2673
Longitudinal hybrid PET/MRI in juvenile-onset Huntington disease (joHD): a pilot study1Neuroscience Research Center, University "Magna Graecia", Catanzaro, Italy, 2Mater Domini University Hospital, Catanzaro, Italy, 3University "Magna Graecia", Catanzaro, Italy, 4IRCCS Casa Sollievo della Sofferenza/CSS-Mendel, San Giovanni Rotondo/Rome, Italy, 5Italian League fo Research on Huntington (LIRH Foundation), Rome, Italy
Synopsis
Hybrid PET-MRI is an emerging technique that allows multimodal evaluation of brain structure and function. This study evaluates longitudinal PET-MRI in one patient with stage-2 joHD, to assess changes related to disease progression. This approach might be useful to test the efficacy of disease-modifying drugs.
Introduction
Juvenile-onset Huntington’s disease (joHD, neurological onset ≤ 20 years) is a rare HD variant associated with large CAG repeat-size alleles ( > 60), which shows different clinical features from adult-onset HD, and typically progresses more rapidly.1 For these reasons, it is crucial to monitor and characterize patterns of disease progression in joHD and compare them to the more studied adult form. In this work, we propose an hybrid-imaging approach for the longitudinal assessment of brain changes in joHD, using multimodal PET-MRI data fusion (i.e., merging information regarding structural, functional and metabolic alterations).Methods
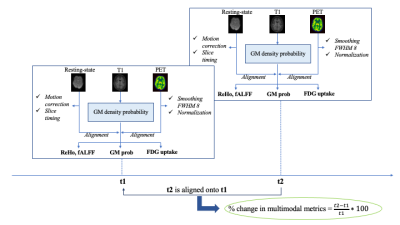
A 20-year-old female with stage-2 joHD, 62 CAG repeats and onset at 16 years, underwent 3T [18F]fluorodeoxyglucose (FDG) PET-MRI (Siemens Biograph mMR) at two timepoints (Oct 2019, with UHDRS-motor score=45 units and Feb 2021, with UHDRS-motor score=53 units). The protocol included the simultaneous acquisition of FDG-PET, T1-weighted MRI and resting-state functional MRI (rs-fMRI).Figure 1 shows the proposed analysis workflow, carried out using FSL and AFNI, applied over single-subject longitudinal data. Briefly, voxel-wise percentage changes at follow-up relative to baseline status were calculated on (i) grey matter (GM) density, (ii) FDG-PET uptake, (iii) rs-fMRI regional homogeneity (ReHo), and (iv) rs-fMRI amplitude of low frequency fluctuation (fALFF).
Results
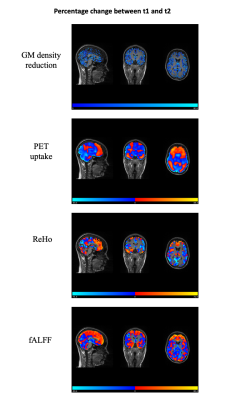
Figure 2 shows voxel-wise maps of percentage changes at follow-up in the single joHD patient. GM density decreased on average over the whole brain of 31.9 ± 21.1% (median [interquartile range] = 28.7 [14.2-47.5]). Percentage change in FDG uptake was increased in the frontoparietal network, while widespread reduction was observed in the remaining regions, including - of note - the thalamus. Maps of ReHo and fALFF longitudinal variations followed the pattern of PET changes, also highlighting increased functional connectivity in the frontal cortex opposed to decreased connectivity in posterior regions.Discussion and Conclusions
Hybrid PET/MRI provides a comprehensive evaluation of complementary imaging characteristics of a single joHD patient. Albeit preliminary, results are promising in characterizing the rapid structural and metabolic changes that affect an adult patient with stage 2 joHD over 18 months.The entity of percentage changes was relevant if compared to known trajectories of healthy aging for the same age range and follow-up period. Furthermore, rates of change resemble the trajectories reported in the literature in advanced adult HD patients,2 and involve specific regions thought to be key hubs affected by this disease.
This pilot study suggests that hybrid imaging might be promising for monitoring individual joHD changes over time. Future developments including a larger number of patients might help gaining further insights into the mechanisms that underlie joHD, shedding light onto whether it is a continuum of the HD spectrum or it has an evolution of its own.
Acknowledgements
No acknowledgement found.References
1. Fusilli, C.; Migliore, S.; Mazza, T.; et al. Biological and clinical manifestations of juvenile Huntington’s disease: a retrospective analysis. Lancet Neuro 2018, 17.11, 986-993.
2. Tabrizi, S.; Scahill, R.I.; Owen, G.; et al. Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington's disease in the TRACK-HD study: analysis of 36-month observational data. The Lancet Neurology 12.7 (2013): 637-649.