2669
In vivo longitudinal characterization of hepatocellular carcinoma based on viscoelasticity and water diffusivity in an orthotopic mouse model1Department of Radiology, Charité Universitätsmedizin Berlin, Berlin, Germany, 2Department of Veterinary Pathology, Free University of Berlin, Berlin, Germany, 3iPATH.Berlin Core Unit of Charité - Universitätsmedizin Berlin, Charité Universitätsmedizin Berlin, Berlin, Germany, 4Institute of Medical Informatics, Charité Universitätsmedizin Berlin, Berlin, Germany
Synopsis
The biophysical properties of hepatocellular carcinoma (HCC) and surrounding liver tissue were investigated longitudinally in a syngeneic, orthotopic mouse model using noninvasive quantitative imaging. In vivo MR elastography (MRE) and diffusion weighted imaging (DWI) were conducted prior to cancer cell implantation and three times during tumor progression. Our preliminary results suggest the involvement of the surrounding liver in terms of changes in viscoelasticity and restricted water diffusion over 6 weeks post implantation, while the HCC appeared to be stiffer and less viscous than the liver at 6 weeks.
Introduction
Hepatocellular carcinoma (HCC) is the 6th most common cancer and the 3rd leading cause of cancer deaths in the world1. Tumor progression is a complex process that involves an intricate interplay between the tumor entity and the Tumor Surrounding Environment (TSE). The interaction between tumor and TSE alters the composition and the structure of the tissue that can be noninvasively characterized by biophysical properties, such viscoelasticity and water diffusivity. Tomoelastography, an multifrequency MR-elastography (MRE) technique2 is an emerging imaging technique for characterizing the mechanical properties of soft tissues in vivo3, 4. The feasibility of MRE for tumor characterization has been demonstrated in organs such as the liver5, 6, brain7, 8 and breast9-12. However, very few MRE studies13-17 addressed longitudinally the biomechanical changes of a tumor during cancer progression with considering the TSE. In this pilot study, we used a syngeneic-orthotopic HCC mouse model18, 19 to investigate the changes of biomechanical properties and water diffusivity in both the tumor and the tumor-bearing liver. The imaging findings were also validated by histopathologic results.Methods
Thirteen adult female BALB/c mice received surgically mouse HCC cells BNL1MEA.7R.1 (ATTC, Manassas, USA) in the liver as described in18. Baseline scans were conducted a week before HCC cell implantation (n=16) and longitudinally at 2 weeks (2w, n=13), 4 weeks (4w, n=8), and 6 weeks (6w, n=4) post-surgery. MRI examinations were performed on 3-T clinical MRI-scanner (Siemens-Lumina) using a small animal coil (Rapid Biomedical, Germany). For tomoelastography, mechanical vibrations of 300, 400 and 500Hz were generated using a costume-made piezo-based actuator system (Figure 1). 3D wavefields were acquired using a single-shot spin-echo echo-planar sequence at eight wave dynamics. Twenty-one 1.2-mm-thick axial slices with resolution of 1.0x1.0 mm2 covering the whole liver were acquired. Diffusion-weighted-imaging (DWI) of 11 axial-slices with voxel size of 0.8x0.8x2.0 mm³ using four b-values (0/50/400/800 s/mm²) were also performed. Tomoelastography data were processed using multifrequency wave-number inversion2 providing maps of c (stiffness, in m/s) and a (penetration rate, inverse viscosity, in m/s), Figure 2. For MRE and DWI, regions of interest (ROIs) were drawn manually using ITK-SNAP20 with reference to T2w anatomical images.For histologic analysis, the mice were sacrificed at different time points and tissue in 2µm sections were stained with hematoxylin and eosin (H&E) to assess cancer progression.
Statistical analysis was conducted with GraphPad Prism (GraphPad-Software, San Diego, California USA). Differences in parameters between timepoints were analyzed using t-test, U-test and Wilcoxon test. p<0.05 was considered significant.
Results
Based on the preliminary data shown in Figure 3, a tendency of liver softening is visible, though not significant (c in m/s, baseline: 3.37±0.35, 2w:3.19±0.34, 4w:3.25±0.30, 6w:3.0±0.24, baseline vs. 6w: p=0.06) with significant increase in wave penetration between 2w and baseline (a, baseline:2.19±0.18 vs 2w:2.04±0.2, p=0.04) and significantly reduced water diffusivity (ADC in 10-3 mm²/s, baseline:1.34±0.43, 2w:1.27±0.31, 4w:1.46±0.24, 6w:0.86±0.39; baseline vs 6w:p=0.01, 2w vs 6w:p=0.04; 4w vs 6w:p=0.02) in 6 weeks. At 6w, tumors were clearly visible in three mice on imaging. Although limited for statistical analysis, comparing to the surrounding liver, all tumors appear stiffer (c_tumor: 3.25±0.19 vs. c_liver: 3.00±0.24 m/s, +12.19%) and less viscous (a: 2.59±0.05 vs. 2.16±0.05 m/s, +19,54%).Histologic analysis on ex-vivo tumor and liver tissue at 6w showed tumor growth with angiogenesis and necrosis. H&E staining of the lung also showed inflammation with alveolar thickening, indicating lung involvement indicating the systemic effects of the disease (Figure 4).
Discussion
To best of our knowledge this is the first in-vivo study for the longitudinal investigation of the biophysical properties of HCC and HCC-hosting liver during cancer progression. Based on our preliminary findings, the HCC-bearing liver presented a tendency of softening with significant early increase in viscosity and reduction in water diffusivity during disease progression. We hypothesize that these changes in viscoelasticity in the liver are associated with the trauma caused by surgery and the subsequence reorganization of cells including necrosis. Furthermore, it might be possible that implanted HCC activate and increase the release of matrix metalloproteinases (MMPs) which degraded the extracellular matrix (ECM) by cleavage of various proteins. Unlike the common HCC-hosting liver in patients that are normally fibrotic or even cirrhotic, in our model, HCC cells were implanted to healthy livers which had significantly less collagen and different ECM composition21-23. Thus, MMPs resulted ECM degradation and structural changes in the liver could be more pronounced leading to whole liver softening. The fragmented proteins could also lead to increased friction, resulting in elevated viscosity. The observed decrease in ADC of the liver could be a result of inflammation-induced global ECM alterations, as reported in in the literature24-26. Although limited by sample size, compared to the surrounding liver, the tumors at 6w appeared to be stiffer, which are consistent with previous studies5, 6, 27. As our data are preliminary, the aforementioned hypotheses need to be validated by further immunohistology analysis.Conclusion
In-vivo biophysical imaging properties, viscoelasticity and water diffusivity, were found sensitive to the structural tissue changes in the liver associated with HCC progression. This early response of HCC-bearing liver might provide information for a better understanding of tumor-TSE interactions.Acknowledgements
We acknowledge support from the German Research Foundation (DFG)- SFB1340 Matrix in Vision and BIOQIC; and the excellent cooperation with the Central Biobank Charité (ZeBanC) which was responsible for digitalization of the histological slides.
References
1. Ferlay J, E.M., Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. 2020 [cited 2021 21.10.2021]; Available from: https://gco.iarc.fr/today.
2. Tzschatzsch, H., et al., Tomoelastography by multifrequency wave number recovery from time-harmonic propagating shear waves. Med Image Anal, 2016. 30: p. 1-10.
3. Muthupillai, R. and R.L. Ehman, Magnetic resonance elastography. Nat Med, 1996. 2(5): p. 601-3.
4. Dittmann, F., et al., Tomoelastography of the abdomen: Tissue mechanical properties of the liver, spleen, kidney, and pancreas from single MR elastography scans at different hydration states. Magn Reson Med, 2017. 78(3): p. 976-983.
5. Hennedige, T.P., et al., Comparison of magnetic resonance elastography and diffusion-weighted imaging for differentiating benign and malignant liver lesions. Eur Radiol, 2016. 26(2): p. 398-406.
6. Venkatesh, S.K., et al., MR elastography of liver tumors: preliminary results. AJR Am J Roentgenol, 2008. 190(6): p. 1534-40.
7. Murphy, M.C., et al., Preoperative assessment of meningioma stiffness using magnetic resonance elastography. J Neurosurg, 2013. 118(3): p. 643-8.
8. Reiss-Zimmermann, M., et al., High Resolution Imaging of Viscoelastic Properties of Intracranial Tumours by Multi-Frequency Magnetic Resonance Elastography. Clin Neuroradiol, 2015. 25(4): p. 371-8.
9. McKnight, A.L., et al., MR elastography of breast cancer: preliminary results. AJR Am J Roentgenol, 2002. 178(6): p. 1411-7.
10. Sinkus, R., et al., High-resolution tensor MR elastography for breast tumour detection. Phys Med Biol, 2000. 45(6): p. 1649-64.
11. Sinkus, R., et al., Imaging anisotropic and viscous properties of breast tissue by magnetic resonance-elastography. Magn Reson Med, 2005. 53(2): p. 372-87.
12. Guo, J., et al., Mechanical characterization of a mouse GL261 glioma model using MR elastography. Proceedings of the 23rd Annual Meeting of ISMRM (ed. I.P.o.t.s.A.M.of ISMRM),Toronto, 2015: p. 1101.
13. Schregel, K., et al., Characterization of glioblastoma in an orthotopic mouse model with magnetic resonance elastography. NMR Biomed, 2018. 31(10): p. e3840.
14. Ahmed, R., et al., Preclinical Imaging Using Single Track Location Shear Wave Elastography: Monitoring the Progression of Murine Pancreatic Tumor Liver Metastasis In Vivo. IEEE Trans Med Imaging, 2020. 39(7): p. 2426-2439.
15. Dizeux, A., et al., In Vivo Multiparametric Ultrasound Imaging of Structural and Functional Tumor Modifications during Therapy. Ultrasound Med Biol, 2017. 43(9): p. 2000-2012.
16. Feng, Y., et al., A longitudinal magnetic resonance elastography study of murine brain tumors following radiation therapy. Phys Med Biol, 2016. 61(16): p. 6121-31.
17. Riegler, J., et al., Tumor Elastography and Its Association with Collagen and the Tumor Microenvironment. Clin Cancer Res, 2018. 24(18): p. 4455-4467.
18. Das, D.K., et al., A "Patient-Like" Orthotopic Syngeneic Mouse Model of Hepatocellular Carcinoma Metastasis. J Vis Exp, 2015(105): p. e52858.
19. Ogunwobi, O.O., et al., Epigenetic upregulation of HGF and c-Met drives metastasis in hepatocellular carcinoma. PLoS One, 2013. 8(5): p. e63765.
20. Yushkevich, P.A., et al., User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage, 2006. 31(3): p. 1116-28.
21. Taouli, B., et al., Magnetic resonance imaging of hepatocellular carcinoma. Gastroenterology, 2004. 127(5 Suppl 1): p. S144-52.
22. Jiang, Y., et al., Inflammatory microenvironment of fibrotic liver promotes hepatocellular carcinoma growth, metastasis and sorafenib resistance through STAT3 activation. Journal of Cellular and Molecular Medicine, 2021. 25(3): p. 1568-1582.
23. Bishayee, A., The role of inflammation and liver cancer. Adv Exp Med Biol, 2014. 816: p. 401-35.
24. Zappa, M., et al., Quantitative MRI in murine radiation-induced rectocolitis: comparison with histopathological inflammation score. NMR Biomed, 2018. 31(4): p. e3897.
25. Taouli, B., et al., Chronic hepatitis: Role of diffusion-weighted imaging and diffusion tensor imaging for the diagnosis of liver fibrosis and inflammation. Journal of Magnetic Resonance Imaging, 2008. 28(1): p. 89-95.
26. Fujimoto, K., et al., Evaluation of the Mean and Entropy of Apparent Diffusion Coefficient Values in Chronic Hepatitis C: Correlation with Pathologic Fibrosis Stage and Inflammatory Activity Grade. Radiology, 2011. 258(3): p. 739-748.
27. Shahryari, M., et al., Tomoelastography Distinguishes Noninvasively between Benign and Malignant Liver Lesions. Cancer Res, 2019. 79(22): p. 5704-5710.
Figures
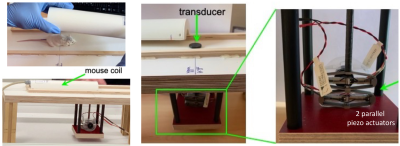
Setup of the in vivo tomoelastography in mice using the small animal/mouse coil and the costume-made piezo-based actuator system.
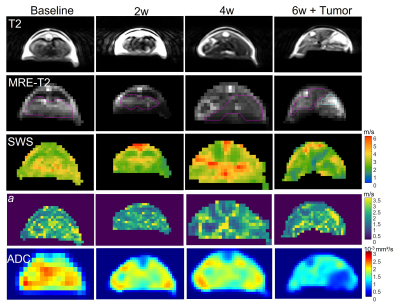
Representative T2w, MRE-T2w, c- and a-maps, ADC map of the liver from mouse#1 at four timepoints. Region of interest (ROI) in liver is outlined in magenta and the tumor in cyan on the MRE-T2w images.
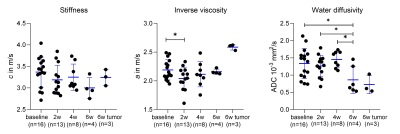
Scatter plots with mean and SD of all measured animals along the timepoints showing stiffness (c in m/s), inverse viscosity (a in m/s) and water diffusivity (ADC in 10-3 mm²/s), significant differences between the timepoints are shown on the graph; * p ≤ 0.05 and ** p ≤ 0.01
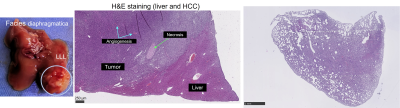
Photo of the tumor bearing liver of the mouse#1 and the corresponding H&E staining of the tumor, the surrounding liver and lung.