2665
Multiparametric MRI assessment of the sex influence in the development and response to anti-inflammatory treatment of a glioblastoma rat model1Instituto de Investigaciones Biomédicas "Alberto Sols", CSIC-UAM, Madrid, Spain, 2Department of Life Sciences and Coimbra Chemistry Centre, Faculty of Science and Technology, University of Coimbra, Coimbra, Portugal
Synopsis
The assessment of sexual differences in glioblastoma growth and therapy response has received little attention. We assessed the influence of sex in the MRI features of an orthotropic GBM rat model submitted to anti-inflammatory therapy. Multiparametric MRI studies were acquired at an early and advanced tumor stage of development. GBMs in untreated males presented a more aggressive pattern – worsened as the tumor progressed – than females. While treated males revealed a positive response to meloxicam treatment, females did not. Our results highlighted that sex is a relevant factor to be considered in GBM outcome and anti-inflammatory therapy success.
Introduction
Glioblastoma (GBM), the most frequent and aggressive of all brain tumors with poor prognosis and survival 1, presents a clear predominance in male humans, but the assessment of sexual differences in its outcome and response to therapy has received little attention, so far.Nonsteroidal anti-inflammatory drugs (NSAIDs) have been studied as anti-tumoral drugs and tested in GBM models with promising results 2. The modulation of inflammation in GBM by selective cyclooxygenase-2 inhibitors not only have a potential therapeutic action, but may also improve responses to other treatments 3,4.
On the other side, magnetic resonance imaging (MRI) parameters are potent tools to be used as non-invasive surrogate biomarkers of this pathology 5, proving to be able of determining the therapeutic response before or during the initial stages of treatment.
In this work we aimed to assess the sexual influence in the MRI tumor features evolution studying GBM-bearing rats treated with a NSAID to investigate its impact on the outcome and therapeutic response.
Methods
GBM was induced in male and female Sprague-Dawley rats by intracranial stereotaxic injection of C6 rat glioma cells. At day 5 post-cell injection, 2 animals’ groups were established: “non-treated” (13 males and 12 females), and “treated” (14 males and 12 females). Non-treated rats were injected subcutaneously with saline and treated animals with the NSAID meloxicam at a dose of 2 mg/kg (1mL/kg/day), during 15 days.MRI acquisitions were carried out in a 7T MRI scanner until day 30 post-cell injection (or until end-point criteria). Tumor volume was followed-up with T2W and Gd-T1W images at different time points. Multiparametric MRI studies included magnetization transfer, diffusion-weighted, T2W and T2*W images, acquired at an early (<100mm3) and an advanced (>130mm3) tumor development stage.
Parametric maps were generated from images with home-made software, and mean values were measured in four regions of interest (ROIs): tumor core, tumor periphery, peritumoral tissue (adjacent to the tumor) and contralateral hemisphere region. Statistical analysis was performed through the unpaired analysis of Student's t test, with two tails and with Welch's correction.
Results
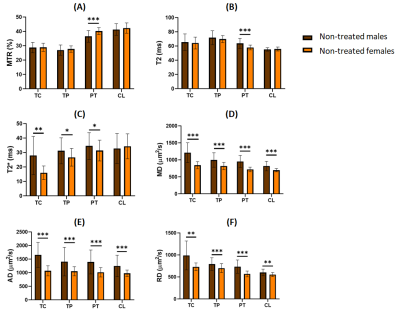
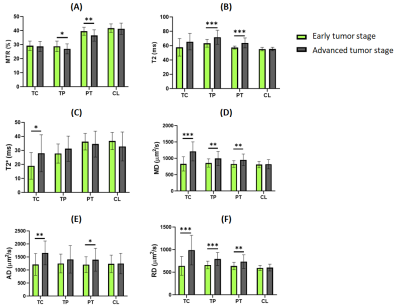
We detected significant differences between untreated males and females in the measures of different parameters and ROIs, both at the early and the advanced tumor stage. We detected in males higher mean-diffusivity (MD), axial-diffusivity (AD) and radial-diffusivity (RD) values in all the ROIs analyzed; higher T2* in all ROIs except the apparently healthy contralateral hemisphere; and lower magnetization-transfer-ratio (MTR) and higher T2 values in peritumoral region (Figure 1).Moreover, several differences were observed between the early and the advanced tumor stages, which were more accentuated in males. By evaluating the tumor development in male rats, we observed an increase in MD, AD and RD values in the tumor-core, tumor-periphery and peritumoral regions; an increase in T2* in the tumor-core; and a decrease in MTR and also an increase in T2 values in the tumor-periphery and peritumoral regions (Figure 2).
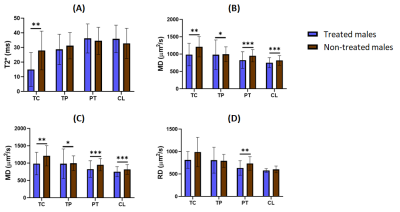
Finally, significant differences were observed in advanced stage tumors growing in male, between treated and untreated groups: both MD and AD values were higher in untreated rats in all ROIs; RD in the peritumoral region; and T2* values in the tumor core region (Figure 3). Interestingly, no differences were observed between treated and untreated females in advanced stage.
Discussion
Our results revealed the presence of greater vasogenic inflammation, related to the increased MD, AD, RD, and T2, and decreased MTR 6-8, and greater angiogenesis suggested by the higher T2* 9 in male GBM bearing rats compared to females.Since peritumoral edema and angiogenesis have been widely associated with tumor aggressiveness and an adverse prognosis in malignant glioma 10, the MRI parameters studied could be considered indicators of a higher aggressiveness and tumor proliferation activity in males than in females, in agreement with previous studies 11. Besides, we detected differences in malignancy related MRI parameters in male rats as the tumors were growing, that were not so significant in female animals when early and advanced stages were compared.
Regarding to the therapy effects, lower MD, AD, RD and T2* in treated males revealed a positive response to the anti-inflammatory treatment in controlling the vasogenic inflammation and tumor-induced angiogenesis, also in agreement with previous studies 2. However, the same effect was not observed in treated females, which indicates that sex is a very important variable to consider in the tumor behavior and the success of therapy, at least regarding NSAIDs as a potential GBM treatment.
Conclusion
The results obtained in this work indicate that the development and therapy of glioblastoma should consider the sex of the individual as a relevant variable once it affects the aggressiveness and therapeutic response, especially in the use of NSAIDs as anti-inflammatory treatment.Acknowledgements
Inês Cabete acknowledges availability, guidance and the scientific knowledge transmitted from Nuria Arias, Maria Guillén, Margarida Castro and Pilar López.References
1. Hanif F, Muzaffar K, Perveen K, et al. Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac J Cancer Prev. 2017;18(1):3-9.
2. Dagêstan Y, Karaca I, Bozkurt ER, et al. Effects of Ibuprofen on orthotopic glioma model in rats. J Coll Physicians Surg Pak. 2012;22(11):690-693.
3. Kang KB, Wang TT, Woon CT, et al. Enhancement of glioblastoma radioresponse by a selective COX-2 inhibitor celecoxib: inhibition of tumor angiogenesis with extensive tumor necrosis. Int J Radiat Oncol Biol Phys. 2007;67(3):888-896.
4. van Nifterik KA, Van Den Berg J, Slotman BJ, et al. Anti-tumour effects by a trimodal combination of temozolomide, meloxicam and X-rays in cultures of human glioma cells. Int J Radiat Biol. 2011;87(2):192-201.
5. Seow P, Wong JHD, Ahmad-Annuar A, et al. Quantitative magnetic resonance imaging and radiogenomic biomarkers for glioma characterisation: a systematic review. Br J Radiol. 2018;91(1092):20170930.
6. Wang S, Zhou J. Diffusion tensor magnetic resonance imaging of rat glioma models: a correlation study of MR imaging and histology. J Comput Assist Tomogr. 2012;36(6):739-744.
7. Lundbom N. Determination of magnetization transfer contrast in tissue: an MR imaging study of brain tumors. Am J Roentgenol. 1992;159(6):1279-1285.
8. Loubinoux I, Volk A, Borredon J, et al. Spreading of vasogenic edema and cytotoxic edema assessed by quantitative diffusion and T2 magnetic resonance imaging. Stroke. 1997;28(2):419-26; 426-7.
9. Barrett T, Brechbiel M, Bernardo M, et al. MRI of tumor angiogenesis. J Magn Reson Imaging. 2007;26(2):235-49.
10. Wu CX, Lin GS, Lin ZX, et al. Peritumoral edema shown by MRI predicts poor clinical outcome in glioblastoma. World J Surg Oncol. 2015;13:97.
11. Pérez-Carro R, Cauli O, López-Larrubia P. Multiparametric magnetic resonance in the assessment of the gender differences in a high-grade glioma rat model. EJNMMI Res. 2014;4(1):44.
Figures