2659
Machine Learning-Based Contrast Enhanced T2-FLAIR Radiomics method for predicting IDH1 Genotype of Diffuse Gliomas1Department of Radiology, The First Affiliated Hospital of Kunming Medical University, Kunming, China, 2Suzhou Institute of Biomedical Engineering and Technology,, Chinese Academy of Sciences, Suzhou, China, 3Fisca Healthcare Ltd, Kumming, China, 4Department of Oncologic Imaging, National Cancer Center,Duke-NUS Graduate Medical School, Singapore, Singapore, 5MR Research, GE Healthcare, Beijing, China
Synopsis
The current study aims to build isocitrate dehydrogenase 1 (IDH1) genotype prediction models based on selected radiomics features derived from contrast-enhanced T2 fluid attenuated inversion recovery (CE-T2-FLAIR) in predicting IDH1 genotype of diffuse gliomas. Radiomics features from CE-T2-FLAIR images go a step further to enrich the content of MRI-based radiomics. It was concluded that machine learning-based radiomics of CE-T2-FLAIR could efficiently predict the IDH1 genotype of diffuse gliomas.
Introduction
Diffuse gliomas account for most of the primary brain tumors in adults. IDH mutation is a critical biomarker for precision diagnosis and prognosis evaluation of adult-type diffuse gliomas [1,2]. In recent years, MRI-based radiomics have been reported to be helpful for IDH1 genotype prediction [3]. Among used MRI sequences, contrast-enhanced T1 weighted imaging (CE-T1WI) greatly contributed to predict IDH1 genotype of diffuse gliomas. The reason may be that images of CE-T1WI reflect the extent of damage to the blood-brain barrier (BBB) in the tumor. CE-T2-FLAIR, which was more sensitive to low concentration contrast agents and showed weakly enhanced lesions more apparent than conventional CE-T1WI [4], has been used in the differential diagnosis of glioma and metastasis, diagnosis of meningioma and meningeal metastasis [5-7]. Nevertheless, no study has been using radiomics features extracted from CE-T2 FLAIR to predict IDH1 genotype of diffuse glioma. This study aims to investigate the value of radiomics models based on CE-T2 FLAIR for predicting IDH1 genotype of diffuse gliomas.Thus, the goal of our research were to build IDH1 genotype prediction models based on selected radiomics features derived from CE-T2-FLAIR and CE-T1WI and evaluate the performance of above models in predicting IDH1 genotype of diffuse gliomas.
Methods
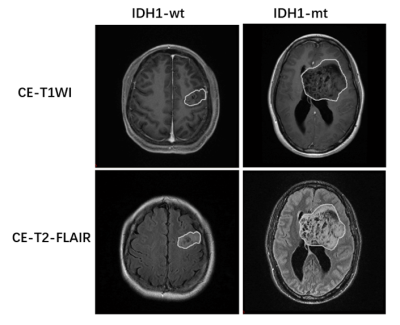
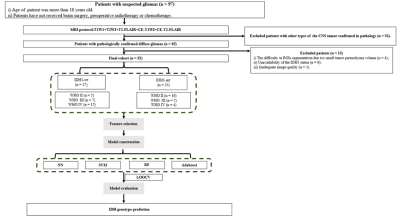
This study was approved by the institutional review board, and written informed consent was obtained from all patients. A total of 52 patients with pathology confirmed diffuse glioma were enrolled for the study, Including 25 cases of IDH1 mutant type (IDH1-mt; WHO grade II / III / IV, 16/5/4) and 27 cases of IDH1 wild type (IDH1-wt; WHO grade II / III / IV, 5/7/15) . All patients underwent CE-T1WI and CE-T2 FLAIR on GE 3.0 T MRI scanner (Discovery MR750w, GE, US) preoperatively. Regions of interest (ROIs) in CE-T1WI and CE-T2-FLAIR images were manually delineated. (see Figure.1). 1134 radiomics features extracted from these ROIs were analyzed. Feature selection was carried out by F-test, mutual information (MI), minimum redundancy maximum relevance (MRMR) and least absolute shrinkage and selection operator (LASSO) Cox regression model. Four types of prediction models, including nearest neighbors (NN), support vector machines (SVM), random forest (RF) and adaptive boosting (Adaboost), were trained and validated using leave-one-out cross validation (LOOCV). The prediction efficiency of each model was evaluated by receiver operating characteristic (ROC) curve. Flow diagram of patient selection and radomics models building were showed in Figure 2.Results
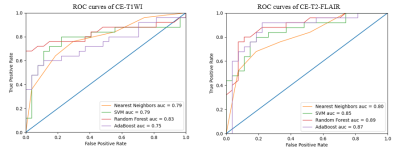
For CE-T1WI, 8 optimal radiomics features were selected by using LASSO Cox regression model, including Gray-level Co-occurrence Matrix (12-1ClusterProminence, 5-4ClusterTendendcy, 11-4ClusterTendendcy), Gradient Orient Histogram (30PercentileArea, 50PercentileArea), Gray-level Run-length Matrix (90LongRunHighGrayLevelEmpha), Shape (MeanBreadth), Intensity Histogram (Kurtosis). For CE-T2-FLAIR, 9 optimal radiomics features were obtained by using the same model, including Gray-level Co-occurrence Matrix (11-4Correlation, 2-4InverseVariance, 1-4MaxProbability, 0-4SumAverage), Gradient Orient Histogram (30PercentileArea), Gray-level Run-length Matrix (90ShortRunHighGrayLevelEmpha), Shape (ConvexHullVolume), Intensity Histogram (Kurtosis, Skewness). Four prediction models (NN, SVM, RF, Adaboost) derived from CE-T1WI or CE-T2-FLAIR can all effectively predict the IDH1 genotype of diffuse gliomas and all prediction models derived from CE-T2-FLAIR had better performance than that from CE-T1WI. AUCs of four models based on radiomics features extracted from CE-T1WI were NN = 0.79 (sensitivity=76.00%, specificity=70.37%), SVM = 0.79 (sensitivity=80.00%, specificity=77.78%), RF = 0.83 (sensitivity=68.00%, specificity=100.00%), Adaboost = 0.75 (sensitivity=60.00%, specificity=88.89%), respectively. AUCs of four models based on radiomics features extracted from CE-T2-FLAIR were NN = 0.80 (sensitivity = 68.00%, specificity = 81.48%), SVM = 0.85 (sensitivity=80.00%, specificity=81.48%), RF = 0.89 (sensitivity=80.00%, specificity=88.89%), Adaboost = 0.87 (sensitivity=92.00%, specificity=77.78%), respectively. ROC curves of different model were showed in Figure 3.Discussion
This study investigated the value of machine learning-based radiomics of CE-T1WI and CE-T2-FLAIR in predicting IDH1 genotype of adult-type diffuse gliomas. It was found that the radiomics features extracted from CE-T1WI and CE-T2-FLAIR mainly included Gray-level Co-occurrence Matrix (GLCM), Gray-level Run-length Matrix (GLRLM), intensity histogram, intensity direct and shape. These radiomics features can describe the regional heterogeneity information of tumor.Four prediction models (NN, SVM, RF, Adaboost) derived from CE-T1WI or CE-T2-FLAIR can all effectively predict the IDH1 genotype of diffuse gliomas and the RF model had the highest prediction efficiency for both CE-T1WI and CE-T2-FLAIR. The Previous study [8] has proved that the superiority of RF model for the prediction of IDH1 genotype of diffuse gliomas. Furthermore, all four prediction models derived from CE-T2-FLAIR had better performance than that from CE-T1WI. Studies have shown that low concentration of contrast agent shortens the T1 relaxation time and results in hyperintensity, while high concentrations of contrast agent reduce the T2 relaxation time and result in hypointensity on T2-FLAIR images. So tissues characterized by smaller take up of contrast agent show greater post-contrast enhancement on CE-T2-FLAIR images than conventional CE-T1WI [4,9]. Jin et al. [10] found that the enhancement degree on CE-T2-FLAIR was negatively correlated with vascular permeability, and the result suggested CE-T2-FLAIR images can better display different degrees of BBB damage in different regions of glioma than CE-T1WI, especially for regions of mild BBB damage.Conclusion
In conclusion, machine learning-based radiomics of CE-T2-FLAIR could predict the IDH1 genotype of diffuse gliomas with high accuracy. Thus, it might be a useful supporting tool in preoperatively predicting the IDH1 genotype of diffuse gliomas, which could aid treatment decision-making and prognosis-evaluating.Acknowledgements
Acknowledgments This work was supported by Yunnan Provincial Science and Technology Department, Kunming Medical University applied basic research (2019FE001(-052)), and Yunnan Provincial Health Science and Technology Program (2018NS0120).References
[1] Weller M, Felsberg J, Hartmann C, et al. Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: a prospective translational study of the German Glioma Network. J Clin Oncol. 2009;27(34):5743-50.
[2] Beiko J, Suki D, Hess KR, Fox BD, Cheung V, Cabral M, et al. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol. 2014;16(1):81-91.
[3] Jiang C, Kong Z, Zhang Y, Liu S, Liu Z, Chen W, et al. Conventional magnetic resonance imaging- based radiomic signature predicts telomerase reverse transcriptase promoter mutation status in grade II and III gliomas. Neuroradiology. 2020;62(7):803-13.
[4] Mathews, V.P., et al., Brain: gadolinium-enhanced fast fluid-attenuated inversion-recovery imaging. Radiology. 1999. 211(1). 257-263.
[5] Buerki Robin, A., et al. An overview of meningiomas. Future oncology (London, England), 2018. 14(21): 2161-2177.
[6] Ercan, N., et al., Diagnostic value of contrast-enhanced fluid-attenuated inversion recovery MR imaging of intracranial metastases. Am J Neuroradiol, 2004. 25(5): 761-765.
[7]Gregory, D., A. Sun, and S. Gregory, Contemporary Endovascular Embolotherapy for Meningioma. Semin intervent Radiol, 2013. 30(3): 263-277.
[8]Shuang Wu, Jin Meng, Qi Yu, et al. Radiomics-based machine learning methods for isocitrate dehydrogenase genotype prediction of diffuse gliomas. Springer Berlin Heidelberg. 2019,145(3): 543-550.
[9] Oguz K K , Cila A . Rim enhancement of meningiomas on fast FLAIR imaging. Neuroradiology, 2003, 45(2):78-81.
[10] Jin T , Ge M , Huang R , et al. Utility of Contrast-Enhanced T2 FLAIR for Imaging Brain Metastases Using a Half-dose High-Relaxivity Contrast Agent. Am J Neuroradiol. 2020, 42(3).