2655
Preferential utilization of acetate in human meningiomas1Neurosurgery, Houston Methodist Hospital and Research Institute, Houston, TX, United States, 2Pathology and Genomic Medicine, Houston Methodist Hospital and Research Institute, Houston, TX, United States
Synopsis
Meningiomas are the most frequently reported central nervous system (CNS) tumors, accounting for 37% of all CNS tumors. Atypical meningiomas (WHO grade-II) show increased cellular proliferation and recurrence. Currently, no chemotherapy is part of the standard of care for meningiomas, and surgery followed by radiotherapy is the mainstay of treatment. Metabolism is the key to many biological processes including tumorigenesis. Unravelling metabolic phenotypes of various histological subtypes of meningiomas will identify new targets for the therapeutic intervention of these tumors. Here, we investigate the in vivo metabolism of [U-13C]glucose and [1,2-13C]acetate in meningioma patients using 13C NMR based isotopomer analysis.
Methods: Meningioma patients were enrolled at the Department of Neurosurgery, Houston Methodist Hospital, following an IRB protocol approved by Houston Methodist Hospital Institutional Review Board. 20% stock solutions of [U-13C]glucose and [1,2-13C]acetate were prepared by a research pharmacist at the investigational drug services (IDS) of Houston Methodist Hospital. On the day of the surgery, intravenous (i.v.) bag containing [U-13C]glucose or [1,2-13C]acetate was delivered to the OR and the patients were infused with either [U-13C]glucose or [1,2-13C]acetate, 2-3 hours prior to the surgical resection of the tumor [9,10]. Blood samples were collected at different time intervals (t = 0, 15, 45, 90, 120 min.) during the infusion and used to calculate plasma enrichment of [U-13C]glucose or [1,2-13C]acetate. Tumor tissues were collected after 120 min of infusion. Both blood and tumor samples were snap-frozen on dry-ice and transported to the laboratory. Blood and tumor tissue specimens were extracted in 5% perchloric acid, supernatants were lyophilized and reconstituted in 180 µL D2O containing 1.0 mM DSS-d6 (pH = 7.4). The reconstituted sample-solutions were transferred to a 3-mm NMR tubes, and 1D 1H and 1H-decoupled 13C NMR data were collected on a Bruker 800 MHz spectrometer equipped with a cryo-probe.
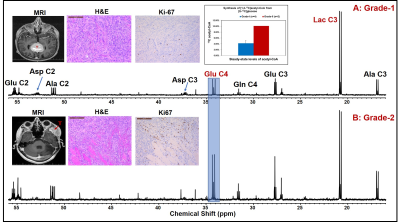
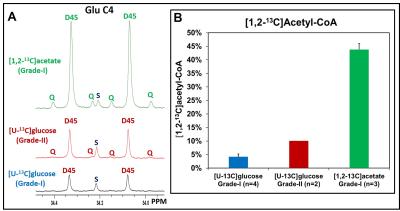
Results: Pathways involved in the metabolism of [U-13C]glucose and [1,2-13C]acetate are schematically shown in Figure 1. Figure 2 shows the 13C NMR spectral comparison of tumor tissue extracts of grade-I (A) and grade-II (B) meningioma patients who were infused with [U-13C]glucose during the surgical resection of tumors. Both grades of tumors showed 13C-13C spin-coupled multiplets in various carbons of glycolytic intermediates lactate (Lac) and alanine (Ala) (i.e. Lac C3 and Ala C3) and TCA cycle metabolites such as glutamate (Glu), glutamine (Gln) and aspartate (Asp) (for example, Glu C3, Glu C4, Gln C4, Asp C2, Asp C3). Levels of Lac C3 were similar in both grades of tumors (Figure 2) whereas Glu C4 which is the readout of the levels of acetyl-CoA are higher in grade-II compared to the grade-I tumors (mean values: 10.10% vs. 4.20 ± 1.02%, inset in upper plot). This suggests that grade-II tumors utilize relatively more glucose than grade-I. Since the amount of acetyl-CoA generated from glucose in meningiomas is only about 10%, these tumors could be utilizing nutrients other than glucose. Acetate is another bioenergetic substrate utilized by tumors [10,11], and we wanted to investigate if meningiomas utilize acetate to fill the bioenergetic gap. We infused meningioma patients with [1,2-13C]acetate and determined the levels of [1,2-13C]acetyl-CoA generated from acetate. Figure 3A shows the comparison of 13C NMR spectral regions of Glu C4 of meningioma tumor extracts from patients infused with [U-13C]glucose (from grade-I and II) and [1,2-13C]acetate (from grade-I), and the chart in Figure 3B shows the levels of [1,2-13C]acetyl-CoA generated from [U-13C]glucose and [1,2-13C]acetate. We can clearly see that meningioma tumors (grade-I) generated 43.83 ± 2.17% of acetyl-CoA from acetate which is ~10 more than that generated from [U-13C]glucose.
Discussion: Although glucose is an abundant nutrient in human body, tumor utilize glucose to a smaller extent and depend on other bioenergetic substrates such as acetate. Most of our patients were from grade-I, we are enrolling more patients and want to include more patients from grade-II to have a better comparison of glucose and acetate metabolism in both groups.
Conclusion: The current study suggest that human meningiomas preferentially utilize acetate. Acetyl-CoA synthetase 2 (ACSS2) is the enzyme responsible for the metabolism of acetate, targeting this enzyme may have therapeutic potential in the treatment of meningiomas.
Acknowledgements
We thank all the patients who participated in this study. This study was supported by the Donna and Kenneth R. Peak Foundation, The Kenneth R. Peak Brain and Pituitary Tumor Treatment Center at Houston Methodist Hospital, The Houston Methodist Foundation, The Taub Foundation, The Pauline Sterne Wolff Foundation, The Veralan Foundation, The Marilee A. and Gary M. Schwarz Foundation, The John S. Dunn Foundation, Contributions in honor of Will McKone. We also thank NMR and Drug Metabolism Core (Baylor College of Medicine, Houston, TX) for providing access to 800 MHz NMR spectrometer.References
References:
1. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011-2015. Neuro Oncol. 2018;20(suppl_4):iv1-iv86.
2. Nowosielski M, Galldiks N, Iglseder S, et al. Diagnostic challenges in meningioma. Neuro Oncol. 2017;19(12):1588-1598.
3. Goldbrunner R, Minniti G, Preusser M, et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17(9):e383-91.
4. Ijare OB, Hambarde S, Brasil da Costa FH, Lopez S, Sharpe MA, Helekar SA, Hangel G, Bogner W, Widhalm G, Bachoo RM, Baskin DS, Pichumani K. Glutamine anaplerosis is required for amino acid biosynthesis in human meningiomas. Neuro Oncol. 2021 Sep 13:noab219.
5. Wishart DS. Is cancer a genetic disease or a metabolic disease? EBioMedicine 2015;2(6):478-479.
6. Vander Heiden MG, DeBerardinis RJ. Understanding the intersections between metabolism and cancer biology. Cell. 2017;168(4):657-669.
7. Faubert B, Solmonson A, DeBerardinis RJ. Metabolic reprogramming and cancer progression. Science. 2020;368(6487):eaaw5473.
8. Monleón D, Morales JM, Gonzalez-Segura A, Gonzalez-Darder JM, Gil-Benso R, Cerdá-Nicolás M, López-Ginés C. Metabolic aggressiveness in benign meningiomas with chromosomal instabilities. Cancer Res. 70 (2010) 8426-8434.
9. E.A. Maher, I. Marin-Valencia, R.M. Bachoo, T. Mashimo, J. Raisanen, K.J. Hatanpaa, A. Jindal, F.M. Jeffrey, C. Choi, C. Madden, D. Mathews, J.M. Pascual, B.E. Mickey, C.R. Malloy, R.J. DeBerardinis, Metabolism of [U-13 C]glucose in human brain tumors in vivo, NMR. Biomed. 25 (2012) 1234-1244.
10. Mashimo T, Pichumani K, Vemireddy V, Hatanpaa KJ, Singh DK, Sirasanagandla S, Nannepaga S, Piccirillo SG, Kovacs Z, Foong C, Huang Z, Barnett S, Mickey BE, DeBerardinis RJ, Tu BP, Maher EA, Bachoo RM. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell. 2014 Dec 18;159(7):1603-14.
11. Schug, Z., Vande Voorde, J. & Gottlieb, E. The metabolic fate of acetate in cancer. Nat Rev Cancer 16, 708–717 (2016).
Figures
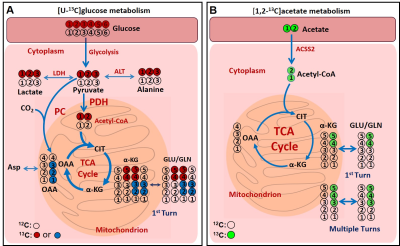
Figure
1:
Schema showing
the metabolism of [U-13C]glucose (A) and [1,2-13C]acetate
(B) in cells. C4 glutamate 13C isotopomers provide the direct readout
of the amount of [1,2-13C]acetyl-CoA produced from [U-13C]glucose
or [1,2-13C]acetate. Abbreviations: Asp, aspartate; CIT, citrate; α-KG, α-ketoglutarate; GLU,
glutamate; GLN, glutamine; OAA, oxaloacetate; LDH, lactate dehydrogenase; ALT,
alanine aminotransferase; PDH, pyruvate dehydrogenase; PC, pyruvate carboxylase.