2653
Influence of Arterial Transit Time Delay in Arterial Spin Labeling on Differentiating Tumor Progression and Pseudo-Progression in Glioblastoma1C. J. Gorter Center for High-Field MRI, Department of Radiology, Leiden University Medical Center, Leiden, Netherlands, 2Department of Radiology, HagaZiekenhuis, Den Haag, Netherlands, 3Department of Radiology, Leiden University Medical Center, Leiden, Netherlands, 4Department of Neurology, Haaglanden Medical Center, Den Haag, Netherlands, 5Department of Neurology, Leiden University Medical Center, Leiden, Netherlands, 6Department of Radiology and Nuclear Medicine, Erasmus MC, University Medical Center, Rotterdam, Netherlands
Synopsis
In the clinical follow-up of glioblastoma patients, presence of delayed arterial transit times (ATT) could affect the evaluation of ASL perfusion data. In this retrospective study the influence of the presence and severity of ATT-artifacts on perfusion assessment and differentiation between tumor progression and pseudo-progression were studied. The results show that the presence of ATT-artifacts lowers the agreement between radiological evaluation of DSC-MRI and ASL, although the severity of ATT-artifacts did not have significant influence. In conclusion, detection of ATT-artifacts is important as it could affect radiological evaluation of ASL-data. Future work aims to include additional quantitative perfusion measures.
INTRODUCTION
Arterial Spin Labeling (ASL) and Dynamic Susceptibility Contrast MRI (DSC-MRI) are commonly used techniques to measure perfusion in patients with glioblastoma. Perfusion MRI is especially helpful in distinguishing tumor progression from pseudo-progression1,2,3,4. The consensus approach for ASL is pCASL with a single post-labeling delay (PLD)5 of 1800ms, in which the resulting images can be affected by arterial transit time (ATT) artifacts6. When the ATT is prolonged, not all labeled spins have arrived in the tissue leading to underestimation of perfusion, which could affect clinical assessment. Therefore, the two research questions of this study are:1. Does the presence of ATT-artifacts impact the assessment of perfusion, i.e. are ASL maps in lesser agreement with DSC-MRI when delayed transit times are present?
2. Do delayed ATTs influence the radiological evaluation of ASL perfusion maps?
METHODS
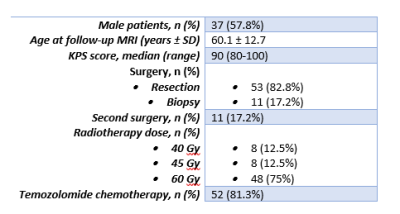
In this retrospective, single-center study data were analyzed from 64 adult patients (Table 1) with histologically confirmed glioblastoma who received postoperative radio(chemo)therapy. The study was performed in accordance with local IRB-regulations. ASL scans were made ±3 months post-radiotherapy either with 2D pCASL with 1600ms labeling-duration (LD) and 1525ms (first slice) – 2120ms (last slice) PLD, or with 3D pCASL 1800ms/1800ms (LD/PLD).The data was evaluated by a neuroradiologist based on three scores:
1. The presence/absence of ATT-artifacts were scored as well as their severity (%).
2. Perfusion score: ASL and DSC perfusion of the enhancing tumor lesion were scored as increased or as normal/decreased. Scores were made per lesion (78 in total), focusing on the nodular part.
3. Radiological score: ASL and DSC perfusion maps were separately interpreted on a 7-point scale with 1 representing definite tumor progression and 7 definite pseudo-progression. The concordance between the DSC and ASL scans in radiological score was tested by a sign test (threshold of p<0.05) both for patients grouped based on presence versus absence as well as on severity of ATT-artifacts (1-50% versus 50-100%, thus excluding patients without ATT-artifacts).
RESULTS
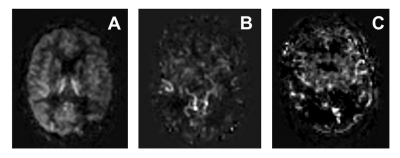
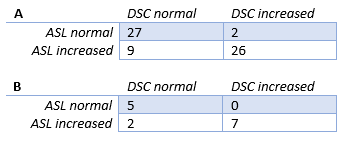
Figure 1 shows example ASL images with different levels of delayed ATT-severity. The total number of patients with ATT-artifacts present was 52 (81.3%) with 14 showing severe ATT-artifacts.Table 2 shows an overview of the perfusion assessment when patients were grouped according to presence/absence of ATT-artifacts.
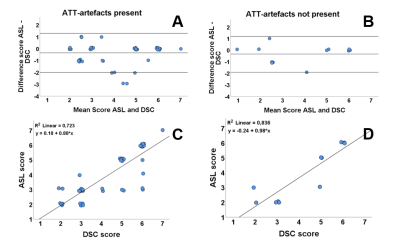
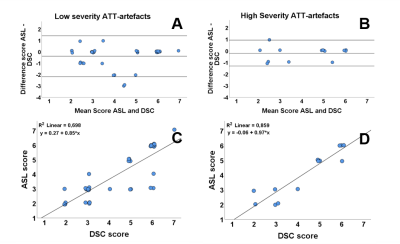
In the presence of ATT-artifacts, for 35 patients (67.3%) the radiological score showed agreement between ASL and DSC. In the absence of ATT-artifacts, this was the case for 7 patients (58.3%). The sign test showed a significant difference in the scoring of the ASL and DSC scans in the presence of ATT-artifacts (p = 0.013, median (IQR) ASL = 3.00 (3.00-6.00), median (IQR) DSC = 5.00 (3.00-6.00)), whereas in the absence of ATT-artifacts no significant difference was found (p = 0.375, median (IQR) ASL = 3.00 (2.00-5.75), median DSC = 4 (2.25-5.75)). Figure 2 shows Bland-Altman and correlation plots for the radiological scores.
For minor ATT-artifacts, exact agreement between ASL and DSC scores was found for 26 patients (68.4%), see Figure 3. For severe ATT-artifacts, this was the case for 9 patients (64.3%). In case of minor ATT-artifacts, a significant difference (p = 0.039, median (IQR) ASL = 3.00 (3.00-6.00), median (IQR) DSC = 5.00 (3.00-6.00)) was found between ASL and DSC scores, while for severe artifacts no significant difference was found (p = 0.375, median (IQR) ASL = 5.00 (2.75-5.25), median (IQR) DSC = 5.00 (3.00-6.00)).
DISCUSSION
In this study the influence of a delayed ATT on the perfusion assessment and radiological evaluation of glioblastoma was investigated. Main findings were: 1) In 81% of the patients ATT-artifacts were present; 2) The perfusion assessment showed reasonable agreement between ASL and DSC with little influence of the presence of ATT-artifacts, although ASL had a minor tendency to overestimate perfusion compared to DSC; 3) The presence, but not the severity of ATT-artifacts affected the radiological evaluation of ASL scans. First, it should be recognized that even when using settings close to the recommended settings, in a large number of patients ATT-artifacts were observed. Second, the relative perfusion scores were for most patients in agreement with DSC-scores, but when in disagreement ASL had the tendency to overestimate perfusion. This finding was, however, independent of the presence of ATT-artifacts. When looking at the radiological interpretation, a statistically significant difference was found between the scoring on DSC and ASL when ATT-artifacts were present. Furthermore, the correlation analysis showed that the ASL evaluation tended slightly more towards tumor progression than for DSC in agreement with the tendency to overestimate perfusion. This could possibly be explained by the apparent increased signal on the ASL scan caused by label present within large arteries. The limited influence of delayed ATT-severity could be explained by the fact that only 14 patients were present in the highest severity group, as opposed to 38 patients with low severity. Furthermore, acknowledgement of severe ATT-artifacts could have caused a bias in the radiological evaluation.CONCLUSION
The presence of delayed ATT in ASL-data seems to impact the radiological evaluation of ASL-data towards tumor progression (as compared to the DSC evaluation), whereas in patients without ATT-artifacts ASL and DSC provide more similar radiological scores. Future work aims to include additional quantitative perfusion data.Acknowledgements
We are thankful to NWO domain AES (project 17079) and Medical Delta Cancer Diagnostics 3.0 for their support.References
1. Fatterpekar GM, Galheigo D, Narayana A, Johnson G, Knopp E. Treatment-related change versus tumor recurrence in high-grade gliomas: A diagnostic conundrum - Use of dynamic susceptibility contrast-enhanced (DSC) perfusion MRI. Am J Roentgenol. 2012;198(1):19-26. doi:10.2214/AJR.11.7417
2. Radenkovic S, Jovanovic M, Stosic-Opincal T, et al. Differentiation between progression and pseudoprogression by arterial spin labeling MRI in patients with glioblastoma multiforme. JBUON. 2017;22(4):1061-1067.
3. van Dijken BRJ, van Laar PJ, Smits M, Dankbaar JW, Enting RH, van der Hoorn A. Perfusion MRI in treatment evaluation of glioblastomas: Clinical relevance of current and future techniques. J Magn Reson Imaging. 2019;49(1):11-22. doi:10.1002/jmri.26306
4. Manning P, Daghighi S, Rajaratnam MK, et al. Differentiation of progressive disease from pseudoprogression using 3D PCASL and DSC perfusion MRI in patients with glioblastoma. J Neurooncol. 2020;147(3):681-690. doi:10.1007/s11060-020-03475-y
5. Alsop DC, Detre JA, Golay X, et al. Recommended Implementation of Arterial Spin-Labeled Perfusion MRI for Clinical Applications: A Consensus of the ISMRM Perfusion Study Group and the European Consortium for ASL in Dementia. doi:10.1002/mrm.25197
6. Grade M, Hernandez Tamames JA, Pizzini FB, Achten E, Golay X, Smits M. A neuroradiologist’s guide to arterial spin labeling MRI in clinical practice. Neuroradiology. 2015;57(12):1181-1202. doi:10.1007/s00234-015-1571-z
Figures