2649
Clinical Feasibility of Brain Tumor Multi-voxel MR Spectroscopic Imaging using Rapid Semi-LASER and EPSI Encoding at 3T1Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Johns Hopkins Medicine, Baltimore, MD, United States, 3A.C.Camargo Cancer Center, Sao Paulo, SP, Brazil, 4GE HealthCare, Munich, Germany
Synopsis
The aim of this study was to evaluate the clinical feasibility of optimized multi-voxel MR spectroscopic imaging (MRSI) at 3T using rapid symmetric echo-planar spectroscopic imaging (EPSI) spatial encoding in patients with brain tumors. We performed the optimized MRSI at 3T using semi-localization by adiabatic selective refocusing pulses (semi-LASER), fast spatial encoding using symmetric EPSI, and robust water suppression with variable power and optimized relaxation delays. A radiologist with extensive experience in neuroimaging and spectroscopy assessed the overall spectral quality. This study is an attempt to integrate high-resolution MRSI in clinical practice for the assessment of brain cancer patients.
Introduction
MR spectroscopic imaging (MRSI) is an imaging technique to assess metabolites concentrations in a specific region of interest in vivo. In brain imaging, Point Resolved Spectroscopy (PRESS) and STimulated Echo Acquisition Mode (STEAM) sequences are used routinely to acquire single-voxel and multi-voxel MRSI. Multi-voxel MRSI enables the simultaneous acquisition of multiple voxels in single or multiple slices. One of the main applications of MRSI in brain cancer is to distinguish between radiation necrosis and recurrence of intraparenchymal tumors, which is necessary to select the appropriate treatment, but it is often difficult based on conventional imaging features alone.1 Additionally MRSI can be valuable in the diagnosis, radiotherapy planning, and assessment of treatment response. In PRESS, refocusing pulses have smaller bandwidths and are thus prone to introducing chemical shift displacement errors. These errors can be reduced with semi-localization using adiabatic pulses (semi-LASER or sLASER)2, where refocusing pulses have broad bandwidths that enable precise volume localization with minimal or no chemical shift displacement errors. In addition, water can be suppressed using a VAPOR scheme.3The clinical applicability of conventional MRSI has been limited due to long acquisition times (>10–15 min) for high resolution spatial metabolic imaging and tissue metabolites detection with higher sensitivity, which increases patient discomfort and increases motion artifacts. Hence, rapid acquisition strategies for MRSI are of great clinical interest.4,5 Symmetric echo-planar spectroscopic imaging (EPSI) has recently been shown to be promising. Preliminary results successfully applied this method in the brain to image spatial metabolite maps with voxel volumes <0.5mL within a clinically feasible time (2 minutes) in phantoms and volunteers.6 The aim of this study was to optimize multi-voxel MRSI at 3T using rapid EPSI spatial encoding in patients with brain tumors and to evaluate the spectral quality from a clinician’s point of view.
Methods
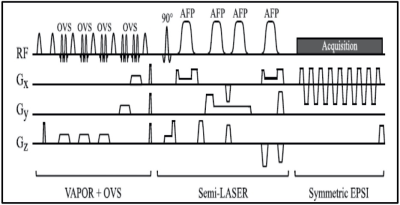
Patients provided written informed consent under an IRB-approved prospective protocol. Experiments were performed in 7 patients with brain tumors (IRB13-239) using a 3T clinical scanner (MR750w, GE Healthcare) and an 8 or 12 channel head coil. Two patients were excluded from MRSI analysis due to non-diagnostic data quality.Anatomical T2-weighted FLAIR (TR/TE=9000/120ms; flip angle=90; 320x256; FOV=24; slice thickness=3mm; acquisition time:3-4min) and single-slice MRSI were performed using the optimized protocol with TR/TE=2s/144ms.6 Prior to the MRSI acquisition, power calibration of VAPOR was performed for each subject to improve water suppression. Water suppressed sLASER-EPSI was performed using a 24x24 matrix under different experimental conditions (Figure 1).
All raw MRSI data and images were transferred to an offline workstation. Data were filtered using 3 Hz exponential filtering, and custom in-house MATLAB code was used to generate MRSI spatial and overlay maps. Further, all voxels of this data were prepared for LCModel fitting analysis using exact Hamiltonian basis sets. Basis sets were generated with 26 brain metabolites including major metabolites such NAA, Cr, Cho, and Lac. Metabolite integrals, absolute quantification, or metabolite ratio maps were generated using in-house software. The accuracy of metabolite measurements was assessed using Cramer Rao Lower Bounds (CRLB). A neuroradiologist reviewed all images to provide the clinical impression and the overall sLASER-EPSI spectral quality, which was categorized as non-diagnostic, fair, good, or excellent. A neuroradiologist assessed images and correlated image findings with clinical outcomes.
Results
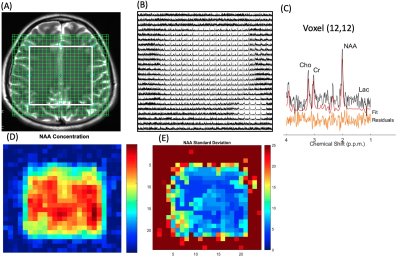
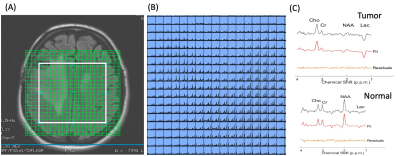
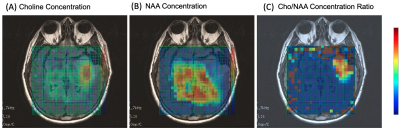
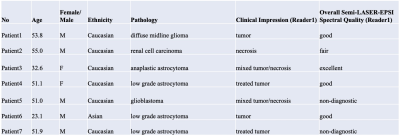
Patients’ characteristics are summarized in Table 1. Figure 2 shows multi-voxel sLASER-EPSI MRSI measurements in a healthy volunteer, indicating uniform spatial localization and accurate estimation of metabolite concentrations such as NAA (standard deviation is < 15% in most of the voxels within the volume of interest indicated in a white rectangle) using this technique. Each voxel was fitted using LCModel as shown in Figure 2C. The scan time was 3 minutes.Figure 3 shows representative multi-voxel sLASER-EPSI MRSI measurements in a patient demonstrating higher Cho and lower NAA in the anaplastic astrocytoma (abnormal FLAIR) compared with normal brain tissue. Additionally, the presence of lactate suggests necrosis within the tumor. This is correlated with clinical findings (Table 1). LCM fits are used for calculating metabolite concentrations.
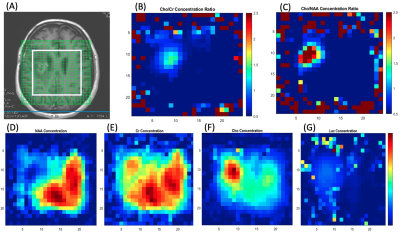
Metabolite concentration color maps were also used to show different metabolite distribution in the tumor and normal tissue (Figures 4 and 5).
Importantly, the MRSI protocol presented here was scored good (3/7) or excellent (1/7). In 1/7 cases, the neuroradiologist found the spectra to be fair, and in 2/7 cases the score was non-diagnostic.
Discussion
Because MRSI scan times in clinical protocols are usually long, there is a need for faster imaging methods. Additionally, at higher magnetic fields like 3T, chemical shift displacements errors are higher and need to be corrected. Our study shows that MRSI acquisition with adiabatic pulses and EPSI spatial encoding may enable clinically feasible scan times to potentially quantify spatial variations of metabolites or to assess response to treatment in patients with brain tumors.Conclusion
sLASER-EPSI multi-voxel MRSI can be used to image 2D/3D spatial metabolite maps within a clinically feasible time in brain cancer patients. In cases where spectra quality is impacted by motion, an additional scan can potentially be acquired while the patient remains in the scanner by adding 3 minutes to the total scan time.Acknowledgements
NIH/NCI P30 CA008748, NIH/NCI UG3 CA239861, BTC Foundation, MSKCC Society grant, and B*CURED Foundation.References
(1) Scheenen TWJ, Klomp DWJ, Wijnen JP, Heerschap A. Short echo time 1H‐MRSI of the human brain at 3T with minimal chemical shift displacement errors using adiabatic refocusing pulses. Magn Reson Med. 2008;59:1‐6.
(2) Tkác I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999;41:649‐656.
(3) Shankar RV, Chang JC, Hu HH, and Kodibagkar VD. Fast data acquisition techniques in magnetic resonance spectroscopic imaging. NMR Biomedicine 2019:32:e4046.
(4) Posse S, DeCarli C, Le Bihan D. Three‐dimensional echo‐planar MR spectroscopic imaging at short echo times in the human brain. Radiology. 1994;192:733‐738.
(5) Coello E, Noeske R, Burns BL, et al. High-resolution echo-planar spectroscopic imaging at ultra-high field. NMR Biomedicine 2018:31:e3950
(6) Thakur SB, Sutton OS, Eduardo J, Noeske R, Lin A, Otazo R, Young R, Rapid acquisition of semi-LASER MR spectroscopic imaging using symmetric EPSI encoding: A preliminary study in phantom and human brain. Proc. Intl. Soc. Mag. Reson. Med. 28 (2020), p2913.
Figures