2646
Structural brain imaging with high-resolution 3D MRI on the Next-Generation 7T brain scanner1Helen Wills Neuroscience Institute, University of California, Berkeley, CA, United States, 2Advanced MRI Technologies, Sebastopol, CA, United States, 3Department of Electrical Engineering and Computer Sciences, University of California, Berkeley, CA, United States, 4Imaging Centre of Excellence, University of Glasgow, Glasgow, United Kingdom, 5MR CoilTech Limited, Glasgow, United Kingdom, 6Radiology, University of California, San Francisco, CA, United States, 7San Francisco Veteran Affairs Health Care System, San Francisco, CA, United States, 8Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 9Harvard-MIT Health Sciences and Technology, MIT, Cambridge, MA, United States
Synopsis
The 128-channel receive, 16-channel parallel transmit capabilities of the new NexGen 7T system, in conjunction with the high amplitude and slew rate of the novel “Impulse” head gradient coil, provide many potential benefits for high-resolution 3D neuroimaging. High channel array coils allow acceleration on two phase encoded axes for very high total acceleration factors, and faster gradients can reduce image readout time for additional benefits. We demonstrate these benefits in different 3D imaging applications (MP2RAGE, FLAIR, FLASH, SWI) at 7T.
Purpose
The Next Generation (NexGen) 7T scanner1, specifically designed and built for brain imaging, incorporates several new features beneficial to high-resolution 3D MR imaging at 7T. The 128-channel receive, 16-channel parallel transmit system allows the use of RF arrays with high-channel counts, leading to increased cortical SNR and reduced g-factor in parallel imaging2. This can be especially beneficial for 3D imaging, where acceleration can be deployed on two phase encoding axes. These high levels of acceleration allow higher resolution images to be collected, and/or the reduction of imaging times for a given resolution, compared to conventional 7T systems.In addition, the newly designed “Impulse” head gradient with high Gmax 200 mT/m and slew rate (SR) 900 T/m/s1,3 can reduce echo spacing and echo train lengths, yielding improvements in some 3D sequences. Comparisons of 3D structural imaging sequences (MP2RAGE, FLAIR, multi-echo FLASH and SWI) are made with the NexGen 7T and a conventional 7T scanner to evaluate useful parallel imaging acceleration factors and useful spatial resolutions and acquisition times.
Methods
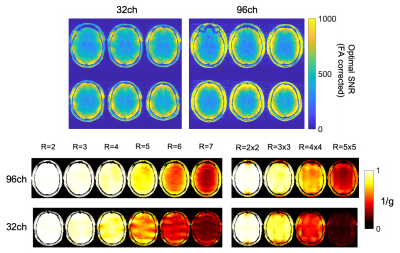
Data were collected on the NexGen 7T designed and built under the NIH BRAIN Initiative1,3. Data were collected using a 16-channel transmit, 96-channel receive RF coil4. Data for comparison were collected on a conventional 7T scanner (MAGNETOM 7T Plus (Siemens Healthcare)) at the SFVA Hospital equipped with a standard 32-channel head coil (Nova Medical) and conventional whole-body gradients (80 mT/m, 200 T/m/s).SNR maps (Figure 1) were calculated via the Kellman method5, and maps were corrected for B1+ using the measured actual flip angle map, scaled to the applied transmit voltage: SNR90 = SNR/(sin α). The coils were combined using the optimized coil combination method that takes into account the noise covariance of the different channels. Parallel imaging encoding capability was also measured by computing g-factors for 1D and 2D in-plane accelerations.
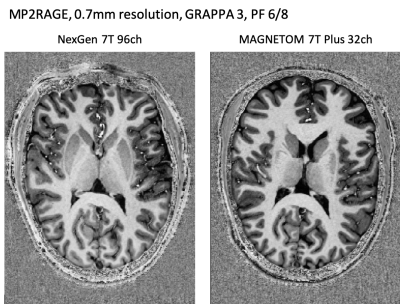
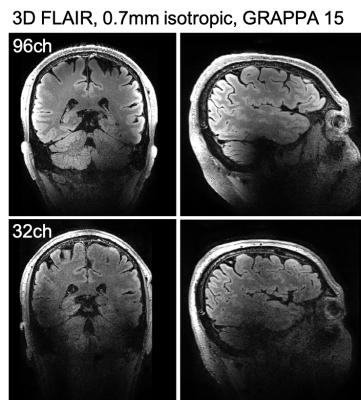
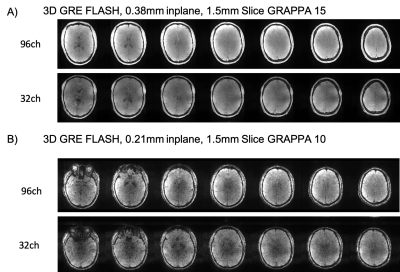
The performance of the coils was assessed on several different 3D sequences using high-acceleration factors: MP2RAGE (Figure 2); FLAIR (Figure 3); FLASH GRAPPA 15 (In-plane resolution 0.38mm, slice thickness 1.5mm,TR 42, minimum TE, BW 400); and FLASH GRAPPA 10 (In-plane resolution 0.21mm, slice thickness 1.5mm, TR 24ms, TE 15ms, BW 120).
Some 3D sequences can also benefit from the improved gradient Gmax and SR of the NexGen to reduce echo spacing and therefore minimize TE and TR. We measured the achievable bandwidth, TR, TE and imaging time for the high resolution, multi-echo FLASH sequence using the Impulse gradients on the NexGen 7T system, and compared them to those achievable using conventional whole-body gradients.
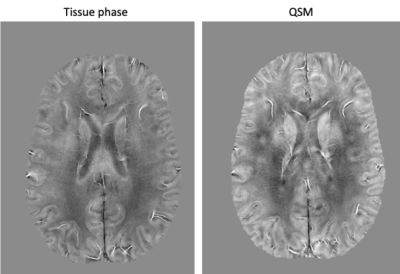
A multi-echo FLASH GRAPPA 10 data protocol was used to create SWI images (Figure 5). Images were reconstructed offline. SENSE6 was performed for coil combination. QSM was calculated with STISuite7.
Results and Discussion
Hardware innovations can produce dramatic improvements in the resolution and quality of structural brain images. The high-channel count head receiver coils on the NexGen system showed the expected gains in cortical SNR with reduced g-factor noise when compared with data collected using a standard 32-channel head coil on standard 7T scanner (Figure 1).For standard accelerations (GRAPPA 3) the MP2RAGE images seem equivalent between the two scanners and RF coils (Figure 2). The ability to apply very high accelerations without a loss in image SNR can be seen from data collected on the 96-channel coil compared to the 32-channel: in the highly accelerated 3D FLAIR (Figure 3) and a 3D GRE FLASH sequence with GRAPPA=15 (Figure 4A) and GRAPPA=10 (Figure 4B). Specifically, the images collected on the 32-channel coil show an increase in noise in the center of the brain, as expected from the g-factor maps. By contrast, the images collected using the 96-channel coil show less of this effect. In addition, the 3D FLAIR image benefits from the more homogeneous B1 field provided by the 16-channel parallel transmit on the RF coil (Figure 2) without the need for dielectric padding. The high-resolution GRAPPA 10 data can also be used to create susceptibility-weighted images (SWI) (Figure 5).
In addition to benefits in SNR and acceleration, the Impulse gradients on the NexGen 7T allow for shorter echo spacings, allowing the readout time of 3D images to be decreased and allowing additional images in multi-echo protocol to be acquired. For 3D FLAIR, the echo spacing reduces from 3.12 to 2.56ms for the 0.7mm protocol here when going from standard body gradients to the Impulse gradients. For the 0.38mm multi-echo 3D FLASH image, the minimum TE for the GRAPPA15 0.38mm image reduced 3.10 to 2.37ms and minimum deltaTE for multi-echo imaging decreased 2.88 to 2.64ms.
Summary and Conclusion
We have demonstrated multiple benefits of the NexGen 7T for 3D imaging in terms of SNR and acceleration from high-channel arrays, and from increases in signal readout speed due to the novel “Impulse” gradients. We expect to see similar or even additional advantages from additional head coils in development for the NexGen system, including a 128-channel receive RF coil8.Acknowledgements
Research supported by the NIH BRAIN Initiative (R01MH111444, U01-EB025162). MATLAB code for g-factor calculations provided by Jonathan R Polimeni.References
1. Feinberg DA, Dietz P, Liu C, Setsompop K, Mukherjee P, Wald LL, Vu AT, Beckett AJS, Insua IG, Schröder M, Stocker S, Bell PH, Rummert E, and Davids M “Design and Development of a Next-Generation 7T human brain scanner with high-performance gradient coil and dense RF arrays.” ISMRM 2021
2. Keil B and Wald LL “Massively parallel MRI detector arrays” JMR 2013
3. Davids M, Dietz P, Ruyters G, Roesler M, Klein V, Guerin B, Feinberg DA, and Wald LL “PNS optimization of a high-performance asymmetric gradient coil for head imaging” ISMRM 2021
4. Gunamony S, Müller R, McElhinney P, Williams SN, Groß-Weege N, Weiskopf N, Möller HE, and Feinberg DA “A 16-channel transmit 96-channel receive head coil for NexGen 7T scanner2021 ISMRM 2021
5. Kelleman P and McVeigh E “Image reconstruction in SNR units: a general method for SNR measurement” MRM 2005
6. Pruessmann JP, Weiger M, Scheidegger MB and Boesiger P “SENSE: sensitivity encoding for fast MRI” MRM 1999
7. STISuite (https://people.eecs.berkeley.edu/∼chunlei.liu/software.html)
8. Gruber B, Stockmann JP, Mareyam A, Keil B, Ghotra A, Feinberg DA, and Wald LL “A 128-Channel head coil array for Cortical Imaging at 7 Tesla” ISMRM 2021
Figures