2644
Dose-dependent response of cerebral blood flow in healthy volunteers following administration of β2-adrenergic receptor agonist clenbuterol1Invicro, London, United Kingdom, 2Center for Clinical Pharmacology, Department of Pharmaceutical and Pharmacological Sciences, KU Leuven, Leuven, Belgium, 3Division of Nuclear Medicine, University Hospital Leuven, Leuven, Belgium, 4Nuclear Medicine and Molecular Imaging, KU Leuven, Leuven, Belgium, 5Department of Neurology, University Hospital Leuven, Leuven, Belgium, 6Centre for Neuroimaging Sciences, Institute of Psychiatry, Psychology and Neuroscience, King’s College, London, United Kingdom, 7CuraSen Therapeutics, San Carlos, CA, United States
Synopsis
Initial evidence is provided for central effects of the β2-AR agonist clenbuterol on CBF in healthy volunteers. Regions showing particular effect (the hippocampus and thalamus) are associated with cognition and neurodegeneration in Parkinson’s and Alzheimer’s Disease, so similar targeting of β2-AR may prove beneficial in these conditions. Further work is required to determine the extent of central mediation of the effect of β2-AR agonism with clenbuterol in healthy volunteers.
Introduction
Early-stage deficiency of the brain’s noradrenergic system is a common pathogenic finding in neurodegenerative disorders including Alzheimer’s and Parkinson’s Disease1, potentially leading to reduced neuronal activity in key limbic and cortical areas (e.g., hippocampus, amygdala, thalamus, and prefrontal cortex). Activation of the noradrenergic system via β2-adrenergic receptor (β2-AR) agonists could therefore offer therapeutic benefit. However, since long-term daily exposure to β2-agonists imparts undesirable peripheral effects (including tachycardia, hyperglycemia and hypokalemia), co-administration of a peripherally restricted β-blocker may protect against adverse events in chronic treatment.Here we focus on cerebral blood flow (CBF) response in healthy volunteers following administration of the β2-AR agonist clenbuterol; both alone, and when co-administered with nadolol – a non-selective β-blocker with minimal brain penetration.
Methods
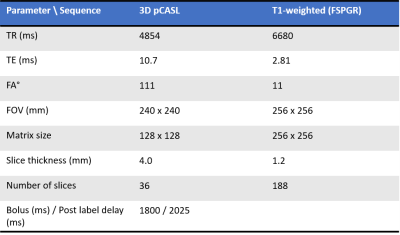
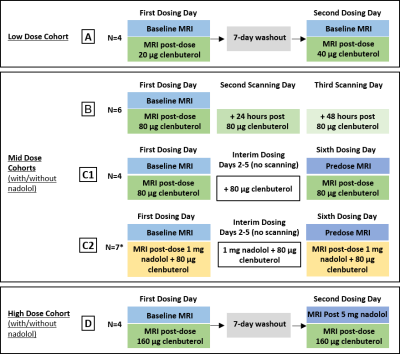
Four independent cohorts of healthy volunteers (N=22 subjects in total, all males, mean age 47.9 years, range 40-55 years) underwent single-delay 3D pCASL and 3D T1-weighted imaging (Figure 1) at baseline, followed by a repeated pCASL scan post administration of clenbuterol (with/without nadolol). Figure 2 provides the imaging and dosing schedule, with imaging performed on a GE Signa PET-MR 3T system.Each subject’s CBF percentage change from baseline following clenbuterol administration was initially calculated and summarised (cohort MEAN±SD) to provide dose response measures in eight pre-specified regions-of-interest (ROIs), including the hippocampus, amygdala and thalamus. To explore the dose-dependent response of CBF following standalone clenbuterol administration, the percentage change from baseline data was pooled together for the relevant cohorts and visits, and similarly summarised (MEAN±SD response for each ROI and dose level). Due to the relatively limited sample size, no further statistical analysis was performed at this stage.
Results
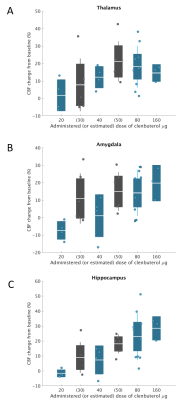
Figure 3 shows the pooled CBF change from baseline data for the thalamus, amygdala, and hippocampus, across the range of clenbuterol doses explored (administered or estimated). There appears to be a dose-dependent response of CBF to clenbuterol, particularly in the hippocampus and thalamus.Looking at immediate (within-day) effect of low-dose clenbuterol (20 µg or 40 µg: cohort A; N=4), there was no obvious central effect in the regions explored: average responses (% change) of -3.8% and 4.5% across the eight grey matter (GM) ROIs, with thalamus responses of 1.8±8.0% and 12.2±5.6%, respectively.
Within-cohort investigations of the mid-dose of clenbuterol (80 µg) indicated (i) from cohort B (N=6): a GM CBF increase post-clenbuterol, with average response in the eight GM ROIs of 17.5%, greatest in the hippocampus (26.7±15.3%) and thalamus (19.8±11.0%); and (ii) from the smaller cohort C1 (N=4): a potential central effect of 80 µg clenbuterol on CBF in two of the four subjects, again, focused in the hippocampus and the thalamus.
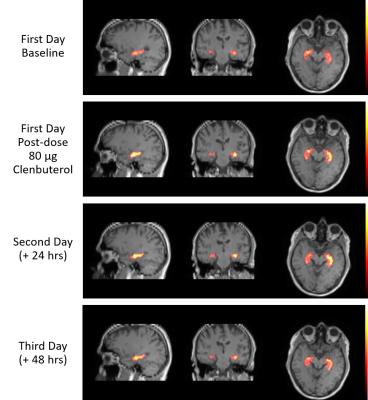
Exploring the prolonged (+24 hrs, +48 hrs) effect of mid-dose clenbuterol (Cohort B, N=6), there was an indication of prolonged (+24 hrs) effect of clenbuterol 80 µg on CBF (Figures 3 and 4), with mean response of 17.2% across the eight GM ROIs, and hippocampus response of 18.0±5.8%. At the cohort level, this prolonged effect is undetectable on day 3 (at +48 hrs postdose), although 2 of the 6 subjects showed potential continued effect on CBF.
Cohort C1 (N=4) indicated a potential benefit of repeated mid-level dosing of 80 µg clenbuterol, with a day 6 postdose CBF response of 15.7% across the eight GM ROIs, thalamus and hippocampus CBF responses of 16.8±8.0% and 20.4±12.6%, respectively. Additional co-administration of 1mg nadolol to this repeated daily dosing regime (cohort C2, N=7) removes the central effect of mid-dose clenbuterol in these healthy volunteers (mean CBF response of 7.9% in the eight GM ROIs at day 6 postdose, with largest positive response in the thalamus, then the hippocampus, but with variability relatively large in this cohort of N=7 subjects).
For the highest dose of 160 µg clenbuterol (cohort D, N=4), CBF response was similar to the mid-dose response without nadolol: mean CBF response was 18.8% across the eight GM ROIs, with highest responses in the hippocampus (28.5±5.8%) and the whole cortex ROI (28.1±17.3%). After controlling for peripheral effects with 5 mg nadolol, the effects of 160 µg clenbuterol on CBF appear to be heavily moderated, with mean response (from first day baseline to second dosing day postdose) of 13.0% across the eight GM ROIs.
Conclusion
Initial evidence is provided for central effects of the β2-AR agonist clenbuterol on CBF in healthy volunteers. Despite the relatively limited number of subjects, the 80 µg and 160 µg doses of clenbuterol showed particular effect on CBF in the thalamus and hippocampus. These regions are associated with cognition and neurodegeneration in Parkinson’s and Alzheimer’s Disease, so similar targeting of β2-AR may prove beneficial in these conditions, as initial work indicates2.Repeated daily co-administration of low-dose (1 mg) nadolol, or pre-administration with a higher dose (5 mg) nadolol, appears to heavily attenuate the central effects of clenbuterol; with greater attenuation compared to our initial work in neurodegenerative patients pre-administered with a single dose of 1 mg nadolol, but with the caveat that the healthy volunteers received a higher overall dose of nadolol.
Overall, these are encouraging results but further investigation with larger cohorts is required to determine the extent of central mediation of the effect of β2-AR agonism with clenbuterol in healthy volunteers.
Acknowledgements
With thanks to the researchers and healthy volunteers who participated in this study.References
1. Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011 Nov;70(11):960-92.
2. Lodeweyckx T, De Hoon J, Van Laere K, et al. Safety, Tolerability and Cerebral Blood Flow After Single Doses of the β2-agonist, Clenbuterol, in Patients with Mild Cognitive Impairment or Parkinson’s Disease. 14th edition of Clinical Trials on Alzheimer’s Disease (CTAD 2021), Boston, November 9-12, 2021.
Figures