2643
A very motion-robust pediatric brain MRI protocol for clinical use
Stefan Skare1,2, Adam van Niekerk1,2, Tim Sprenger2,3, Aleksandra Ramolli1, Yords Österman1, Jan Svoboda1, Ola Norbeck1,2, Henric Rydén1,2, Enrico Avventi1,2, and Johan Berglund2
1Karolinska University Hospital, Stockholm, Sweden, 2Karolinska Institutet, Stockholm, Sweden, 3GE Healthcare, Stockholm, Sweden
1Karolinska University Hospital, Stockholm, Sweden, 2Karolinska Institutet, Stockholm, Sweden, 3GE Healthcare, Stockholm, Sweden
Synopsis
Selected pediatric patients (3-9Y) scheduled for a clinical brain MRI under general anesthesia are enrolled in this ongoing study to be scanned awake, while watching a movie for entertainment, using a new motion-robust brain MRI protocol. The protocol consists solely of custom-made, inherently motion-robust, sequences, with all necessary MR contrasts for clinical use. All but one sequence uses real-time correction of head motion with an external tracking device. All eight unsedated pediatric patients scanned so far resulted in diagnostic images, despite some patients moving their heads in excess of 30 degrees (peak-peak) during the exam.
Background
The level of motion artifacts in unsedated pediatric brain MRI is expected to be high (1). Therefore, it is common to do a brain MRI exam for these patients under full general anesthesia (GA), just for the purpose of artifact-free diagnostic images. The use of GA adds hours of preparation, wake-up times and prolongs the MRI door-to-door time. GA is also not entirely risk-free. Non-acute patients usually have to wait several months, up to a year, to get an MRI under GA due to limited anesthesia resources at our hospital. Prospectively motion-corrected (PMC) brain MRI is an active research field. To avoid GA, the motion robust protocol needs to include all the MRI contrasts necessary for the brain exam. In addition, 3D Cartesian MRI, even with ideal prospective correction (with no latency or error) is not guaranteed to yield ghost-free images when the motion is large, as the image phase changes in the brain as the head moves, leading to inconsistencies in k-space. To be robust against large head motion, we propose performing PMC using external hardware devices and combining it with fast single-shot imaging methods that capture the field state such as PROPELLER and EPI-based pulse sequences, which have the additional benefit of retrospective correction.Methods
For a comprehensive generic pediatric brain MRI protocol to be useful for most patients, it was decided that the necessary MR contrasts, pulse sequences, and scan planes were as shown in the table (Fig. 1). The protocol begins with a newly developed NeuroMix sequence, producing 9 contrasts in ~150 s. While NeuroMix is not yet compatible with PMC, it is mostly based on 2D single-shot EPI and FSE readouts (except for two 3D-EPI modules), with retrospective correction intra- and inter- contrasts. The remaining scans were of higher resolution and image quality compared to NeuroMix, consisting of T2w, T1-FLAIR, and T2-FLAIR PMC-enabled PROPELLER (2) in different scan planes, as well as axial PMC-enabled DW-EPI (3) and 2D sequential SWI EPI scans (4). All sequences in this protocol were developed in-house using the KS Foundation programming framework (5). The prospective motion tracking device (TracInnovations, Denmark) comprises a "3D" camera shooting an infrared point cloud on the patient's face to map its shape and to perform real-time motion updates fed back to the running sequence. All scans were performed on a 3T SIGNA Premier GE Healthcare using a 48-channel head coil, and eight unsedated pediatric patients (4-8Y), originally scheduled for a clinical MRI under GA, have been scanned so far with the protocol.Results
All eight patients (3-8Y) remained comfortable during the entire examination as they were able to watch cartoon movies via a custom-made rear-facing mirror that was designed to be compatible with the point cloud camera placed outside the head coil (Fig. 2a). The ability to remain still varied from about three degrees (peak-peak) over the examination to over 30 degrees (peak-peak) in both the nodding and the left-right directions (Fig. 3). MR images from the first patient (first row in Fig. 3) are shown in Fig. 4. This was the only patient so far with a pathology (intracranial encephalocele). A similar layout of the MR images is shown in Fig. 5 for the patient that moved the most (row six in Fig. 3).Discussion
The amount of head motion was not correlated with age, but more so by how eager the patient was to interact with their nearby parent or by what happened in the movie being played. Even though patient #1 (Fig. 4) did not move nearly as much as patient #6 (Fig. 5), the corrupted T1w 3D-EPI (23 s scan time) for patient #1, but not #6, illustrates how unpredictable Cartesian 3D scans are. In summary, we have found that relying on a combination of PMC and retrospectively corrected inherently motion-robust 2D sequences allows diagnostic brain MRI with head motion up to the point that is possible inside the RF coil, which is over an order of magnitude more than what becomes problematic in conventional MRI.Acknowledgements
No acknowledgement found.References
- Copeland A, Silver E, Korja R, et al. Infant and Child MRI: A Review of Scanning Procedures. Front. Neurosci. 2021;15:666020.
- Norbeck O, van Niekerk A, Avventi E, et al. T1 -FLAIR imaging during continuous head motion: Combining PROPELLER with an intelligent marker. Magn. Reson. Med. 2020 doi: 10.1002/mrm.28477.
- Berglund J, van Niekerk A, Rydén H, et al. Prospective motion correction for diffusion weighted EPI of the brain using an optical markerless tracker. Magn. Reson. Med. 2021;85:1427–1440.
- Berglund J, Sprenger T, van Niekerk A, et al. Motion-insensitive susceptibility weighted imaging. Magn. Reson. Med. 2021;86:1970–1982.
- Skare S, Avventi E, Norbeck O, Ryden H. An abstraction layer for simpler EPIC pulse programming on GE MR systems in a clinical environment. In: Proceedings of the 25th Annual Meeting of ISMRM, Honolulu, Hawaii. ; 2017. p. 3813.
Figures
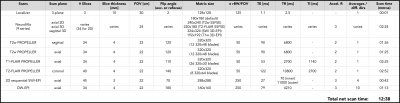
Sequence specifications for the motion robust brain MRI protocol, shown here for 34 slices. The leading NeuroMix scan (2:24 min) acquires 9 series in one go, mostly consisting of 2D single-shot SSFSE and EPI readouts with two additional 3D-EPI modules (SWI, T1w). For the remaining sequences in the protocol, prospective motion correction was performed using a point-cloud-based 3D camera system. All scans were based on either PROPELLER or 2D single-shot EPI to be robust against phase effects from shot to shot. In-plane retrospective motion correction was performed throughout.
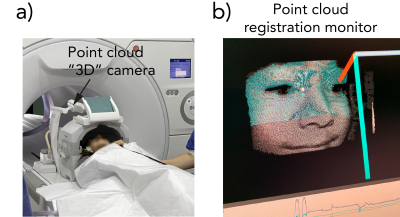
a) Photo of patient #1 in position (c.f. Fig. 4) to be examined with the proposed protocol, with the point-cloud-based 3D camera monitoring the patient's head movements with real-time feedback to the active pulse sequence. b) The tracking computer that calculates the amount of motion with a total latency of < 0.1 s.
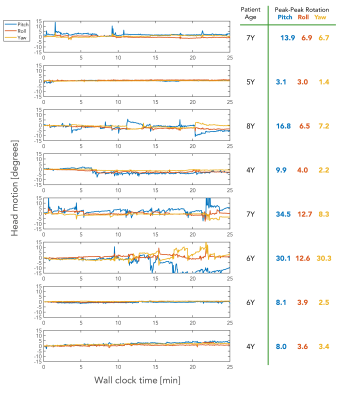
Head rotations of the eight pediatric patients scanned so far, continuously recorded with the tracking system during the entire exam including dead time for pauses and patient communication (wall clock time of 25 min). Note the 30-degree scale on the y-axes. The most compliant patient (#2) moved its head about 3 degrees during the scan, which would have resulted in ghosted conventional Cartesian MR images. Patient #6 moved about 30 degrees along both the pitch (nodding) and yaw (left-right) directions. Corresponding images are shown in Fig. 5.
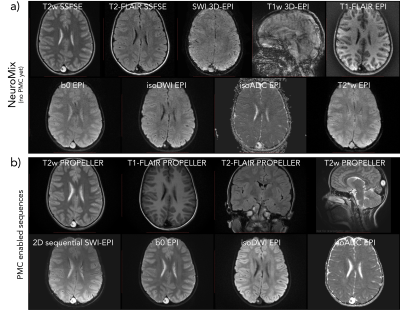
MR images for the first patient (7Y), diagnosed with an intracranial encephalocele in the posterior part of the brain. a) The NeuroMix scan, producing 9 image contrasts, two of which are motion-sensitive albeit fast 3D-EPI. In this case, the patient moved enough to negatively affect both 3D-EPI blocks (SWI and T1w), while the other 2D contrasts were diagnostic. b) PMC-enabled high-res scans based on PROPELLER and SS-EPI. Note that the 2D sequential SWI-EPI acquires one plane in ~0.2 s, freezing phase effects due to head motion.
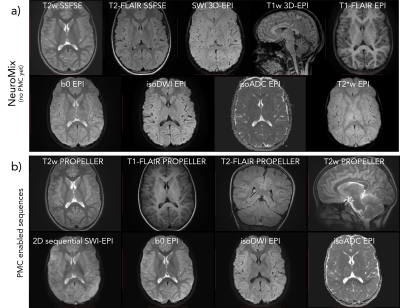
MR images for patient #6, moving the most so far, with the same layout as Fig. 4. a) NeuroMix b) the PMC-enabled high-res sequences. Even with this extreme amount of head motion, the combined use of PMC, retrospective correction, inherently motion robust sequences, and some MR contrast redundancy, resulted in diagnostic images. Similar to all other patients scanned, this patient did not need to do the original MRI under GA later on.
DOI: https://doi.org/10.58530/2022/2643