2640
Advancing Concussion Assessment in Pediatrics (A-CAP): Longitudinal changes in brain metabolites in pediatric concussion1Radiology, University of Calgary, Calgary, AB, Canada, 2Pediatrics, University of Alberta and Stollery Children's Hospital, Edmonton, AB, Canada, 3Pediatrics, University of British Columbia, Vancouver, BC, Canada, 4Psychology, University of Montreal and St Justine Hospital, Montreal, QC, Canada, 5Pediatrics and Emergency Medicine, University of Ottawa and Children's Hospital of Eastern Ontario, Ottawa, ON, Canada, 6University of Calgary, Calgary, AB, Canada, 7Psychology, University of Calgary, Calgary, AB, Canada
Synopsis
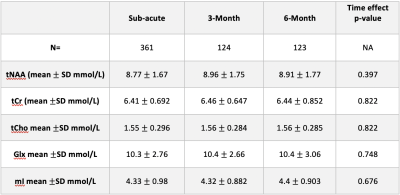
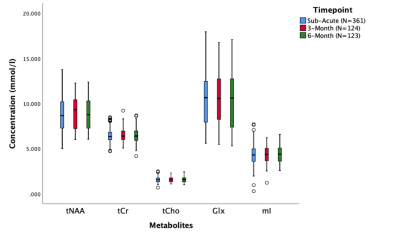
Disruptions in brain metabolites following concussion are commonly reported in the literature. However, studies have typically included only one timepoint, studied adults and/or had limited sample size. In the largest MRS dataset in pediatric concussion to date, we show that metabolites do not differ significantly over time points with longitudinal data collected sub-acutely (<14 days post-injury) and at 3- or 6-months follow-up. This lack of change may indicate that metabolites are relatively stable, that changes are regionally specific or occur more acutely, or there are subgroups that need to be specifically considered.
Introduction
Millions of children in North America sustain concussion annually (1). Although research in concussion is increasing, there is still little understanding of the pathophysiology. Magnetic resonance spectroscopy (MRS) was used to explore several key metabolites that may serve as biomarkers. Previous studies suggest tNAA (N-Acetyl Aspartate + N-Acetyl Aspartyl-Glutamate, a marker of neuronal integrity) is decreased following concussion (2–4). Other metabolites such as, Glx (Glutamate + Glutamine, a marker of excitatory tone), tCho (Glycero-Phosphocholine + Choline + Phosphocholine, indicates membrane turnover), tCr (Creatine + Phosphocreatine, indicates bioenergetics), and mI (Inositol + Glycine, a marker of biomembranes) increase following concussion (4–9).Prior work has primarily studied adults with concussion and has been limited by small sample sizes. In the largest pediatric MRS-concussion dataset acquired to date, we examined brain metabolites with longitudinal data collected 2-weeks post-injury and follow-up at 3- or 6- months post-injury.
Methods
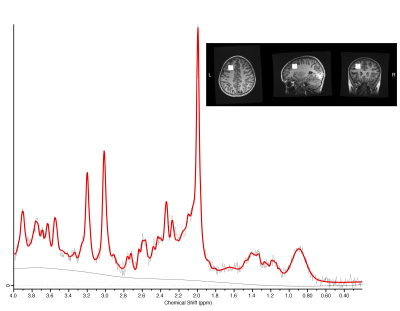
Children (age 8-16.99 y) with concussion were recruited across five emergency departments as part of the Advancing Concussion Assessment in Pediatrics study (10). 3T magnetic resonance imaging including proton MRS was conducted at a mean of 12 days (1.5-33 days) post-injury. Participants were then randomized to either a 3- or 6-month follow-up MRI. MRS data were collected using a short-echo single voxel PRESS (TE/TR=30/2000ms, 8ml voxel, 96 averages) from the left dorsolateral prefrontal cortex (figure 1).MRS data were preprocessed in FID-A (11), quantified in LCModel (12), and subsequently tissue corrected (13). An example spectrum is shown in Figure 1. Data quality was assessed by visual inspection, signal-to-noise (SNR) ratio, and a Cramer-Rao lower bound threshold criterion of 20% was applied.
Separate linear mixed effects models were used to study each metabolite (tNAA, tCr, tCho, Glx, mI) over the 3 timepoints (sub-acute, 3-month, and 6-month). This model was chosen to account for individuals receiving either a 3 or 6-month follow-up scan. Analyses controlled for the effects of age, sex, and site/vendor (combination of different sites and vendor, GE/Siemens).
Results
Data were acquired from 361 concussion participants at the sub-acute period, 124 at the 3-month period and 123 at the 6-month period.There were no significant differences in tNAA, tCr, tCho, Glx, and mI across time (p>0.05 for all metabolites, Figure 2, Table 1). Age was a significant factor; tNAA significantly increased with age while Glx significantly decreased with age (p<0.0001). Sex was also a significant factor, females had significantly lower Glx (p<0.01).
Discussion
We found no change in metabolites (tNAA, tCr, tCho and Glx and mI) over time in pediatric concussion between the sub-acute, 3- and 6- month time points. This suggests that overall metabolites do not change in this phase post-concussion. Alternatively, changes may only become apparent when considering specific subgroups that are not identified in this study.As the age range in this study encompasses an important developmental period in pediatrics, where metabolites are known to change (14), it is not be surprising to see age as a significant factor for tNAA and Glx. Interestingly, Glx is also significantly affected by sex.
Previous literature has consistently shown tNAA acutely decreased following concussion compared to controls (2–4), but a longitudinal study did not find any effect of time on NAA in WM in concussion (5). Conversely, some recent work (15) in pediatric concussion has shown that metabolites are not significantly decreased in the subacute phase compared to OI controls. Another recent study suggests decreases in tNAA are driven by decreases in NAAG (16). As gray matter has less NAAG than white matter, the mixed voxel tissue-content in the current study may have limited the ability to detect change in tNAA.
Similarly, studies documenting the change in tCr and tCho have been in white matter regions (5,8,17). Glx may be differentially affected by tissue as literature suggests Glx increased in white matter (5,18) and decreased in gray matter (5,8) with concussion. mI has been shown to increase (6), however, it has also been shown to have no effect in global GM and WM (19). Future studies should consider examining different regions and tissue type to best understand these effects.
Many previous studies are clustered in specific patient populations for example sport-related concussion, and the smaller samples may have homogeneous populations leading to population specific changes. Additionally, the adult brain may show different metabolite responses compared to the more plastic developing brain. Future work may also consider comparing patients with persistent symptoms to those who recover. As the effect of time on metabolites was only studied within the concussion group to determine concussion specific effects, future work may consider time effects in a control population as a reference.
Significance
This is the largest MRS dataset of pediatric concussion. No changes in metabolites were found between sub-acute, 3 months and 6 months post-injury. We suggest voxel tissue content is a significant factor to consider and subgroups may show different progressions but need to be clearly identified.Acknowledgements
No acknowledgement found.References
1. Faul M, Xu L, Wald MM, Coronado VG. Traumatic Brain Injury in the United States: Emergency department visits, hospitalizations and deaths 2002-2006. Centers Dis Control Prev Natl Cent Inj Prev Control. 2010;
2. Vagnozzi R, Signoretti S, Cristofori L, et al. Assessment of metabolic brain damage and recovery following mild traumatic brain injury: A multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain. 2010;133(11):3232–3242. doi: 10.1093/brain/awq200.
3. Veeramuthu V, Seow P, Narayanan V, et al. Neurometabolites Alteration in the Acute Phase of Mild Traumatic Brain Injury (mTBI): An in Vivo Proton Magnetic Resonance Spectroscopy (1H-MRS) Study. Acad Radiol. Elsevier Inc.; 2018;25(9):1167–1177. doi: 10.1016/j.acra.2018.01.005.
4. Johnson B, Zhang K, Gay M, et al. Metabolic alterations in corpus callosum may compromise brain functional connectivity in MTBI patients: An 1H-MRS study. Neurosci Lett. Elsevier Ireland Ltd; 2012;509(1):5–8. doi: 10.1016/j.neulet.2011.11.013.
5. Yeo RA, Gasparovic C, Merideth F, Ruhl D, Doezema D, Mayer AR. A longitudinal proton magnetic resonance spectroscopy study of mild traumatic brain injury. J Neurotrauma. 2011;28(1):1–11. doi: 10.1089/neu.2010.1578.
6. Kierans AS, Kirov II, Gonen O, et al. Myoinositol and glutamate complex neurometabolite abnormality after mild traumatic brain injury. Neurology. 2014;82(6):521–528. doi: 10.1212/WNL.0000000000000105.
7. Shutter L, Tong KA, Holshouser BA. Proton MRS in acute traumatic brain injury: Role for glutamate/glutamine and choline for outcome prediction. J Neurotrauma. 2004;21(12):1693–1705. doi: 10.1089/neu.2004.21.1693.
8. Gasparovic C, Yeo R, Mannell M, et al. Neurometabolite concentrations in gray and white matter in mild traumatic brain injury: An 1H-magnetic resonance spectroscopy study. J Neurotrauma. 2009;26(10):1635–1643. doi: 10.1089/neu.2009.0896.
9. Lin AP, Liao HJ, Merugumala SK, Prabhu SP, Meehan WP, Ross BD. Metabolic imaging of mild traumatic brain injury. Brain Imaging Behav. 2012;6(2):208–223. doi: 10.1007/s11682-012-9181-4.
10. Yeates KO, Beauchamp M, Craig W, et al. Advancing Concussion Assessment in Pediatrics (A-CAP): A prospective, concurrent cohort, longitudinal study of mild traumatic brain injury in children: Protocol study. BMJ Open. 2017;7(7):1–14. doi: 10.1136/bmjopen-2017-017012.
11. Simpson R, Devenyi GA, Jezzard P, Hennessy TJ, Near J. Advanced processing and simulation of MRS data using the FID appliance (FID-A)—An open source, MATLAB-based toolkit. Magn Reson Med. 2017;77(1):23–33. doi: 10.1002/mrm.26091.
12. Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14(4):260–264. doi: 10.1002/nbm.698.
13. Near J, Harris AD, Juchem C, et al. Preprocessing, analysis and quantification in single‐voxel magnetic resonance spectroscopy: experts’ consensus recommendations. NMR Biomed. 2020;(December 2019):1–23. doi: 10.1002/nbm.4257.
14. Blüml S, Panigrahy A. MR spectroscopy of pediatric brain disorders. MR Spectrosc Pediatr Brain Disord. 2013;9781441958:1–401. doi: 10.1007/978-1-4419-5864-8.
15. La PL, Walker R, Joyce JM, et al. Exploring Brain Metabolites in Pediatric Concussion: An A-CAP Abstract Program # 2225. Int Soc Magn Reson Med. 2021.
16. Menshchikov P, Ivantsova A, Manzhurtsev A, et al. Separate N-acetyl aspartyl glutamate, N-acetyl aspartate, aspartate, and glutamate quantification after pediatric mild traumatic brain injury in the acute phase. Magn Reson Med. 2020;84(6):2918–2931. doi: 10.1002/mrm.28332.
17. Sarmento E, Moreira P, Brito C, Souza J, Jevoux C, Bigal M. Proton spectroscopy in patients with post-traumatic headache attributed to mild head injury. Headache. 2009;49(9):1345–1352. doi: 10.1111/j.1526-4610.2009.01494.x.
18. Schranz AL, Manning KY, Dekaban GA, et al. Reduced brain glutamine in female varsity rugby athletes after concussion and in non-concussed athletes after a season of play. Hum Brain Mapp. 2018;39(4):1489–1499. doi: 10.1002/hbm.23919.
19. Kirov II, Tal A, Babb JS, Lui YW, Grossman RI, Gonen O. Diffuse axonal injury in mild traumatic brain injury: a 3D multivoxel proton MR spectroscopy study Ivan. J Neurol. 2013;260(1):242–252. doi: 10.1007/s00415-012-6626-z.
Figures