2634
Virtual precision surgery for mri implant safety1Advanced Materials Metrology and Life Science, Istituto Nazionale di Ricerca Metrologica, Torino, Italy, 2IRCCS Istituto Ortopedico Rizzoli, Bologna, Italy
Synopsis
In silico evaluation of heating during an MRI exam for patients with passive implants patients need detailed anatomical models to reproduce in vivo scenarios. A quantification of the influence of the model approximation is fundamental to define the main requirements of the digital model.Here, we analyze the impact of different model approximation in simulating RF and gradient-induced heating caused by orthopedic implants. It was found that, whereas the heating due to switched gradient fields does not strictly require the adoption of advanced virtual surgery, the heating caused by RF is strongly affected by the precision of the digital model.
INTRODUCTION
Safety concerns of patients with passive implants undergoing MRI exams have increased in relevance, also at standardization level1-3. To this purpose, reliable and validated tools, capable of reproducing in vivo scenarios, are essential. Whereas several studies have focused on active implantable medical devices4-11, greater attention has been recently given to passive implants, due to evident heating effects produced by radiofrequency (RF) and switched gradient coil (GC) fields12-15.In this work, the impact of increasingly detailed virtual surgery on predicting RF and GC heating is analyzed. Modified versions of two anatomical models belonging to the Virtual Population (ViP)16 are analyzed using four levels of details: from a single compartment model to a model obtained by applying a thorough virtual surgery procedure. In all cases, commercial hip, knee and shoulder implants are adopted.
METHODS
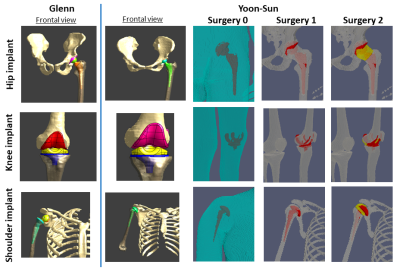
Heating analysis for RF and GC were evaluated for four levels of details of the modified anatomical models: a homogeneous anatomical model (Surgery 0), with physical properties of the ASTM F2182 gel17; a heterogeneous anatomical model with implants overlapped to the native tissues (Surgery 1); two anatomical models with realistic modification of the tissues surrounding the implant according to the virtual surgery procedure (Surgery 2). Two filler tissues are considered in the latter: connective tissue (2A) or synovial fluid (2B), with properties of the cerebrospinal fluid assigned due to tissue similarities18,19.The adult female Yoon-Sun and the adult male Glenn, from ViP Version 3, were selected. A total hip (model Apta-Fix) and total knee (model Genus MB) prostheses (Adler Ortho® SpA, Italy), were considered and their sizes selected according to the anatomy. Regarding the shoulder implant, two products were selected: an anatomichemi-prosthesis (model SMR Anatomic) for Yoon-Sun and a reverse prosthesis (model SMR Reverse) for GlennLimaCorporate SpA, Italy).
Virtual implants were implanted on one side using Sim4Life V5.220, modifying the original posture of the human models when required. For virtual Surgery 2, implant position was double-checked by orthopedic surgeons at IOR making special care to shape the periprosthetic bones of the human model to optimally fit the relevant implants (Figure 1).
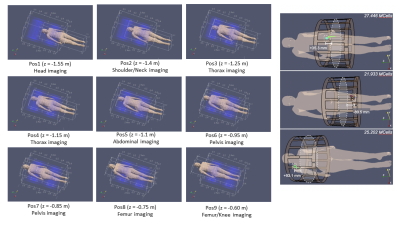
RF simulations were performed with Sim4Life, positioning the virtual model within birdcage coils, tuned at 1.5 and 3 T, so that the implant is placed at the coil extremities from the isocenter (Figure 2) to maximize the power deposition12,15. The coil input power was adjusted to generate an average B1+ of 2 μT on a 2D transverse slice at the coil isocenter.
For the GC simulations, a tubular gradient coil set was used, considering sequences producing worst-case exposure15: EPI and TrueFISP. A homemade software was used21-23, analyzing nine body positions within the bore.
RESULTS
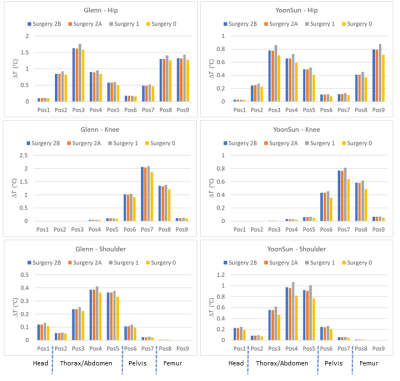
For the GC heating with EPI sequence (frequency encoding along the z-axis) the maximum temperature increases after 900 s are collected in Figure 3. Due to the smaller size, the temperature increases for hip and knee in Yoon-Sun are lower with respect to Glenn. For the shoulder implant, values found for Glenn are significantly lower, due to the non-metallic glenosphere.For the hip implant, values range from 0.88 °C for Yoon-Sun (resp. 1.77 °C for Glenn), with Surgery 1 to 0.72 °C (resp. 1.6 °C), with Surgery 0. For the knee implant, values range from 0.81 °C (resp. 2.1 °C), with Surgery 1 to 0.64 °C (resp. 1.9 °C), with Surgery 0. For the shoulder implant values range from 1.1 °C (resp. 0.41 °C), with Surgery 1 to 0.82 °C (resp. 0.36 °C), with Surgery 0. Surgery 1 always gives rise to the highest values, whereas results obtained with Surgery 2A and 2B are almost coincident.
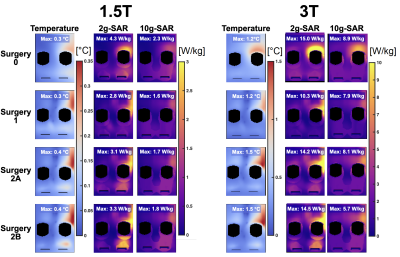
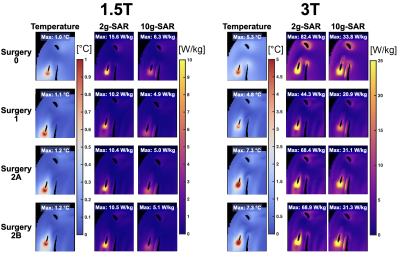
Figures 4 and 5 summarize temperature, 2-g and 10-g SAR results of Yoon-Sun for the knee implant (lowest temperature increase) and the shoulder implant (greatest temperature increase).
At 1.5 T, the maximum temperature increase was observed for Surgeries 2A and 2B (hip: 0.87 °C; Knee: 0.36 °C; Shoulder: 1.2 °C), followed by Surgery 1 and Surgery 0. At 3 T, the maximum temperature was observed for Surgeries 2A and 2B for the knee and shoulder implants (Knee: 1.6 °C; Shoulder: 7.4 °C), followed by Surgery 0 and Surgery 1. Maximum temperature for the hip implant was observed for Surgery 1 (3.6 °C), followed by Surgeries 2A/2B and Surgery 0.
DISCUSSION AND CONCLUSIONS
GC-induced heating does not strictly need the adoption of advanced virtual surgery. A simple overlapping of the implant CAD with the body anatomy successfully provides an acceptable estimation of heating. Surgery 2 results are weakly affected by the selected filling material. This overall behavior was found to be almost independent of the anatomical model and considered sequence.For the RF-induced heating, an accurate virtual surgery is found to be essential. The different surgeries give rise to a variation up to ~55 % both for the 2-g averaged SAR and the maximum temperature increase. The differences are mainly due to the electromagnetic problem in the tissues, which dominates the RF-induced heating. The properties of the filling material negligibly affect the computed SAR distribution in Surgery 2.
Acknowledgements
The results presented here have been developed in the framework of the 17IND01 MIMAS Project. This project has received funding from the EMPIR Programme, co-financed by the Participating States and from the European Union’s Horizon 2020 Research and Innovation Programme.
Authors thank Zurich Med Tech, Zurich, Switzerland for making the modified versions of the ViP models, equipped with the considered orthopedic implants, available in the voxelized structure for subsequent in silico analysis.
CAD models of hip and knee implants were kindly provided by the manufacturer of prosthetic devices Adler Ortho® SpA, Italy (www.adlerortho.com).
Authors wish to thanks the orthopaedic surgeons F. Giardina, E. Tassinari (Orthopaedic-Traumatology and Prosthetic surgery and revisions of hip and knee implants, Istituto Ortopedico Rizzoli, Italy) and E. Guerra (Shoulder and Elbow Surgery, Istituto Ortopedico Rizzoli, Italy) for supervising virtual surgery activities.
References
1. OECD/EU (2016), Health at a Glance: Europe 2016 – State of Health in the EU Cycle, OECD Publishing, Paris. Available via http://dx.doi.org/10.1787/9789264265592-en. Accessed 14 June 2021.
2. Lukas Winter L., Seifert F., Zilberti L., Murbach M. and Ittermann B. MRI-Related Heating of Implants and Devices: A Review. J. MAGN. RESON. IMAGING 2020.
3. ISO_TS_10974_2018(E) - Assessment of the safety of magnetic resonance imaging for patients with an active implantable medical device.
4. Nordbeck P., Weiss I., Ehses P., Ritter O., Warmuth M., Fidler F., Herold V., Jakob P. M., Ladd M.E., Quick H.H., and Bauer W.R. Measuring RF-Induced Currents Inside Implants: Impact of Device Configuration on MRI Safety of Cardiac Pacemaker Leads. Magnetic Resonance in Medicine 61:570–578 (2009).
5. Mattei E., Triventi M., Calcagnini G., Censi F., Kainz W., Mendoza G., Bassen H.I. and Bartolini P. Complexity of MRI induced heating on metallic leads: Experimental measurements of 374 configurations. BioMedical Engineering OnLine 2008, 7:11.
6. Mattei E., Calcagnini G., Censi F., Triventi M., and Bartolini P. Numerical Model for Estimating RF-Induced Heating on a Pacemaker Implant During MRI: Experimental Validation. IEEE TRANSACTIONS ON BIOMEDICAL ENGINEERING, VOL. 57, NO. 8, AUGUST 2010.
7. McElcheran C.E., Golestanirad L., Iacono M.I., Wei P.S., Yang B., Anderson K.J.T., Bonmassar G., Graham S. J. Numerical Simulations of Realistic Lead Trajectories and an Experimental Verification Support the Efficacy of Parallel Radiofrequency Transmission to Reduce Heating of Deep Brain Stimulation Implants during MRI. Scientific Reports, (2019) 9:2124.
8. Golestanirad L., Angelone L.M., Iacono M.I., Katnani H., Wald L.L. and Bonmassar G. Local SAR near Deep Brain Stimulation (DBS) Electrodes at 64 and 127 MHz: A Simulation Study of the Effect of Extracranial Loops. Magnetic Resonance in Medicine 78:1558–1565 (2017).
9. Guerin B., Serano P., Iacono M.I., Herrington T.M., Widge A.S., Dougherty D.D., Bonmassar G., Angelone L.M. and Wald L.L. Realistic modeling of deep brain stimulation implants for electromagnetic MRI safety studies. Phys. Med. Biol. 63 095015, 2018.
10. Iacono M.I., Makris N., Mainardi L., Angelone L.M., and Bonmassar G. MRI-Based Multiscale Model for Electromagnetic Analysis in the Human Head with Implanted DBS. Computational and Mathematical Methods in Medicine Volume 2013, 694171.
11. Jeong H., Ntolkeras G., Alhilani M., Atefi S.R., Zöllei L., Fujimoto K., et al. Development, validation, and pilot MRI safety study of a high-resolution, open source, whole body pediatric numerical simulation model. PLoS ONE 16(1), 2021.
12. Powell J., Papadaki A., Hand J., Hart A., McRobbie D. Numerical simulation of SAR induced around Co-Cr-Mo hip prostheses in situ exposed to RF fields associated with 1.5 and 3 T MRI body coils. Magn Reson Med 2012; 68:960–968.
13. Seo Y., Wang Z.J. Measurement and evaluation of specific absorption rate and temperature elevation caused by an artificial hip joint during MRI scanning. Scientific Reports, (2021) 11:1134.
14. Destruel A., Fuentes M., Weber E., O’Brien K., Jin J., Liu F., Barth M., Crozier S. A numerical and experimental study of RF shimming in the presence of hip prostheses using adaptive SAR at 3 T. Magn Reson Med. 2019; 81:3826–3839.
15. Arduino A., Zanovello U., Hand J., Zilberti L., Brühl R., Chiampi M., Bottauscio O. Heating of hip joint implants in MRI: The combined effect of RF and switched-gradient fields. Magn Reson Med., 85 (6), 3447-3462, 2021.
16. Gosselin M.C., Neufeld E., Moser H., Huber E., Farcito S., Gerber L., Jedensjö M., Hilber I., Di Gennaro F., Lloyd B., Cherubini E., Szczerba D., Kainz W. and Kuster N. Development of a new generation of high-resolution anatomical models for medical device evaluation: the virtual population 3.0. Phys. Med. Biol. 59 2014, 5287–303.
17. ASTM F2182-11a: Standard test method for measurement of radio frequency induced heating on or near passive implants during magnetic resonance imaging. ASTM International, West Conshohocken, PA, USA, 2019.
18. Hasgall P.A., Di Gennaro F., Baumgartner C., Neufeld E., Lloyd B., Gosselin M.C., Payne D., Klingenböck A., Kuster N. IT’IS Database for thermal and electromagnetic parameters of biological tissues, Version 4.0, May 15, 2018, DOI: 10.13099/VIP21000-04-0. Itis.swiss/database.
19. Moghadam M.N., Abdel-Sayed P., Camine V.M., Pioletti D.P. Impact of synovial fluid flow on temperature regulation in knee cartilage. Journal of Biomechanics 2015; 48:370-374.
20. Sim4Life, Computable human phantoms, Zurich MedTech AG, Zurich https://zmt.swiss/sim4life/.
21. Arduino A., Bottauscio O., Brühl R., Chiampi M., Zilberti L. In silico evaluation of the thermal stress induced by MRI switched gradient fields in patients with metallic hip implant. Phys Med Biol 2019;64:245006.
22. Bottauscio O., Chiampi M., Hand J., Zilberti L. A GPU computational code for eddy-current problems in voxel-based anatomy. IEEE Trans Magn 2015; 51:1-4.
23. Arduino A., Bottauscio O., Chiampi M., Zilberti L. Douglas–Gunn method applied to dosimetric assessment in magnetic resonance imaging. IEEE Trans Magn 2017; 53:1-4.
Figures