2633
Assessment of Femur Metallic Implant Safety at 3T1Aix Marseille Univ, CNRS, Centrale Marseille, Institut Fresnel, Marseille, France, 2Aix Marseille Univ, CNRS, CRMBM, Marseille, France, 3Aix Marseille Univ, CNRS, ISM, Marseille, France, 4Multiwave Imaging, Marseille, France
Synopsis
We assessed electromagnetic behavior of metallic implant used for distal femur fracture in typical MRI situation. |B1+| field, SAR and temperature variations were computed at 3T using a surface coil on human model for different positions of the implant relative to the coil. To validate the simulation, we measured the E-field and compared it to simulated E-field. |B1+| field maps showed an interesting augmentation near the implant. Both global SAR and local SAR levels proved that it is possible to safely image bone repair. However, temperature elevation near the tip of the implant was important and is to be considerate.
Introduction
Osteosynthesis is a surgical procedure commonly used for complex fractures with the aim of repairing broken bones. Titanium (Ti) implants are used in femur fractures to constrain bone during the healing process1. MRI is a high-interest technique because of its non-invasive aspect and its spatial and temporal resolutions2. However, the presence of implants can be problematic as magnetic interactions between the implant and radiofrequency (RF) field can cause tissue heating3,4. Safety guidelines indicate that the global SAR (globSAR) must be under 2W/kg5. Given that globSAR does not provide information related to potential local hotspots6. Local SAR (locSAR) must be calculated in order to assess possible heating near the tip of the implant7. Investigating temperature is also an important topic of ongoing research that demonstrated that a globSAR of 2.5W/kg could lead to temperature elevation up to 9°C after 15 minutes of exposure in phantom8. In the present study, we performed electromagnetic simulations to assess the behavior of the femur implant at 3T. More specifically, we quantified globSAR, locSAR averaged over 10g of tissues (SAR10g), and computed temperature. A phantom study was performed to compare and validate the results of simulations and experiments using E‐field measurements.Methods
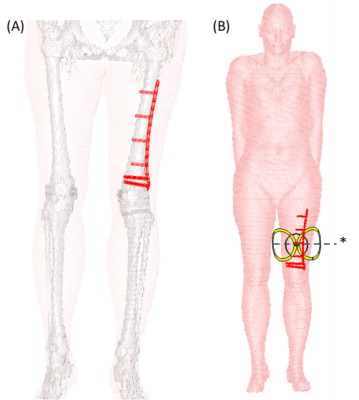
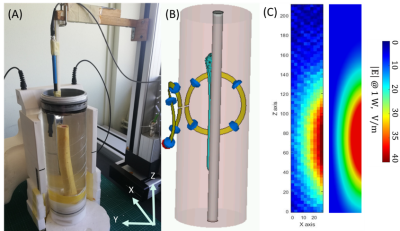
Numerical modeling based on finite-difference-time-domain method was used to calculate the RF induced E-field and |B1+| field distributions, compute globSAR and locSAR at 3T with a transceiver flexible surface coil in a human model with a Ti implant (CST Microwave Studio 2019). The female model from the human voxel family was modified to include the femur implant and screws were added to have realistic simulation (Fig. 1). A transient time-domain solver was used for thermal simulations9. The core temperature was assumed to be constant, the initial body temperature was set at 37°C and the ambient temperature was 20°C. The heat transfer coefficient of 3.2W/m2/K was computed to estimate heat losses due to radiation and convection on the skin. Temperature changes were computed for 15 minutes of simulation time. Four different coil positions were investigated i.e. ventral, dorsal, right, and left to conclude on the best setting for the safest MRI acquisition. As part of the validation procedure, a cylindrical phantom was designed and constructed to measure E-field distribution to compare to the simulation results. The phantom was filled with a solution (σ=0.5 S/m; εr=78 at 128MHz) where the implant was secured by Ti screws to the femur sawbone (Fig. 4). E-field probe was immersed in the phantom and 2D maps were acquired. Measurements were performed over the implant length (210mm) and width (27mm) with a 3mm step. The E-field and |B1+| field distributions from simulations and measurements were scaled to 1W of input power.Results
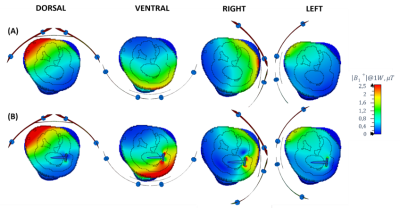
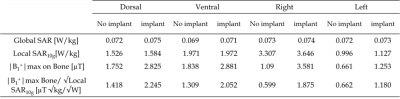
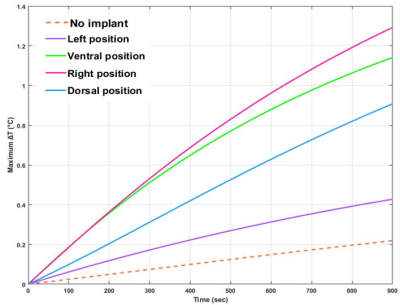
As displayed in Fig.2, the simulated |B1+|field maps illustrated an increased field close to the implant for both ventral (3.835±0.135µT) and right (3.505±0.126µT) positions. The GlobSAR levels were under 2W/kg for the different positions. The lowest SAR averaged over 10g of tissue was identified for the left position with a value equal to 1.127W/Kg. The |B1+|maximum Bone/√LocSAR10g ratio illustrated in Table 1 indicated that the optimal coil performance was obtained for the dorsal position (2.245µT/√kg/W). As plotted in Fig. 3, the largest ΔTmax elevation near the implant after 15 minutes of continuous exposure to RF, was significant for the right position (1.32°C). Temperature variations for the left and dorsal positions were lower (0.42 and 0.93°C, respectively) and there were no significant changes observed without the implant.Discussion
As MRI can be of interest in order to assess bone repair, |B1+| field, globSAR, locSAR, and temperature changes near metallic implants should be robustly quantified. The present globSAR and locSAR measurements demonstrated that MRI in presence of a femur implant is possible within the human safety recommendations. However, the temperature elevation measured in the different conditions showed that this variation is to be considered. Overall, these parameters helped us gain insights on the best position of the coil vs. implant to safely monitor bone consolidation. GlobSAR and locSAR failed to predict peak temperatures after RF exposure duration10,11. The robustness of SAR measurements is tightly linked to the accuracy of electrical and thermal properties of tissues and their thermoregulation model. Directly measuring E-field helps validate results of simulation because the probe is not affected by the presence of implant making measurements accurate.Conclusion
This work showed a simulation case of RF application in implant and metrics measurements to ensure respect of MRI safety guidelines at 3T. Both results from globSAR and locSAR showed that they are not reliable to predict RF heating near the femur implant, this was exhibited by temperature variation up to 1.32°C when SAR measurements respected the guidelines. Directly measuring E-field in phantom provides fast and accurate validation of simulation results. Our future work will include measuring temperature variation in phantom with the implant at 3T using a fiberoptic temperature probe. Finally, to state the safety of MRI on patients with implants, field strength, RF coil, scan conditions, and implant specifications must be considered.Acknowledgements
This project has received funding from the European Union's Horizon 2020 research and innovation program under grant agreement No 952106 (M-ONE project); and from the Excellence Initiative of Aix-Marseille University - A*MIDEX, a french "Investissements d'Avenir" program under Multiwave chair of Medical Imaging.References
1. C Krettek, M Müller, T Miclau. Evolution of Minimally Invasive Plate Osteosynthesis (MIPO) in the femur. Injury. 2001;32:3.
2. J Fritz, B Lurie, TT Miller, HG Potter. MR Imaging of Hip Arthroplasty Implants. Radiographics. 2014;34:4:106 - 132.
3. Winter L, Seifert F, Zilberti L, Murbach M and Ittermann B. MRI-Related Heating of Implants and Devices: A Review. J Magn Reson Imaging. 2021;53: 1646-1665.
4. Tokaya JP, Raaijmakers AJE, Luijten PR, Bakker JF, van den Berg CAT. MRI-based transfer function determination for the assessment of implant safety. Magn Reson Med. 2017;78(6):2449-2459.
5. International Electrotechnical Commission. Medical electrical equipment. Part2: Particular requirements for the basic safety and essential performance of magnetic resonance equipment for medical diagnosis. Geneva, Switzerland: IEC; 2010.
6. Graesslin I, Homann H, Biederer S, et al. A specific absorption rate prediction concept for parallel transmission MR. Magn Reson Med. 2012;68:1664–1674.
7. Powell J, Papadaki A, Hand J, Hart A, McRobbie D. Numerical simulation of SAR induced around Co‐Cr‐Mo hip prostheses in situ exposed to RF fields associated with 1.5 and 3 T MRI body coils. Magn Reson Med. 2012;68:960–968.
8. Muranaka H, Horiguchi T, Ueda Y, Usui S, Tanki N, Nakamura O. Evaluation of RF heating on hip joint implant in phantom during MRI examinations. Nihon Hoshasen Gijutsu Gakkai Zasshi. 2010;66:725–733.
9. Pennes HH. Analysis of tissue and arterial blood temperatures in the resting human forearm. Appl Physiol. 1948;1:93-122.
10. Oberacker E, Paul K, Huelnhagen T, et al. Magnetic resonance safety and compatibility of tantalum markers used in proton beam therapy for intraocular tumors: a 7.0 Tesla study. Magn Reson Med. 2017;78:1533–1546.
11. Destruel A, O'Brien K, Jin J, Liu F, Barth M, Crozier S. Adaptive SAR mass‐averaging framework to improve predictions of local RF heating near a hip implant for parallel transmit at 7T. Magn Reson Med. 2019;81:615–627.
Figures