2631
Implant heating in muscle-mimicking phantoms: an in silico evaluation of perfusion cooling1Medical Biophysics, Western University, London, ON, Canada, 2The xMR Labs, Western University, London, ON, Canada, 3Physics & Astronomy, Western University, London, ON, Canada
Synopsis
We have developed a novel perfusion phantom and this work describes the simulated RF-heating of a 10 cm titanium rod inside this phantom when placed inside the current gold standard (ASTM) phantom, and our geometry/muscle-mimicking phantoms. Simulations show a 30-45% decrease in observed heating due to perfusion cooling, and once these simulations are experimentally validated they could pave the way for improved acceptance criteria and ultimately improved patient access to MRI
Introduction
Currently, the state-of-the-art standard for measuring radiofrequency-induced heating in implanted medical devices (IMDs) is described in ASTM F2182-19a1. In this method, IMDs are placed in a rectangular phantom and exposed to RF to determine how much they heat, then compared to an acceptance criterion to determine if the implant/patient can safely be scanned. The ASTM phantom is filled with a gelled saline with an electrical conductivity and relative permittivity that represent “the average human body tissue”1. Further, this phantom intentionally neglects perfusion/convection cooling (by including a gelling agent) which further exaggerates IMD heating beyond what would happen in the body. In ISO 10974; a more comprehensive device test standard aimed at active implants, it is recommended that implants are tested in an “appropriate phantom filled with media that simulates the tissues that dominate the immediate surroundings of the implant”2. The ASTM phantoms’ lack of realistic geometry, tissue, and perfusion mimicking leads to some IMDs performing poorly in the heating test (e.g. orthopedic devices) which can negatively impact patient access to MRI. In reality, perfusion should prevent these devices from reaching such temperatures, but it is not feasible to quantify perfusion cooling inside patients with IMDs. Thus, we must simulate and experimentally validate the effect of tissue and perfusion mimicking on IMD heating. Improving our understanding of perfusion cooling and its’ impact on IMD heating will allow us to improve acceptance criterion without sacrificing patient safety.Methods
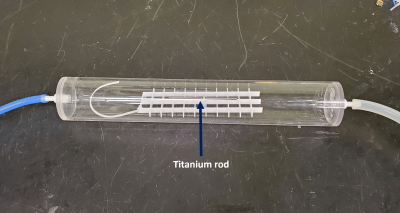
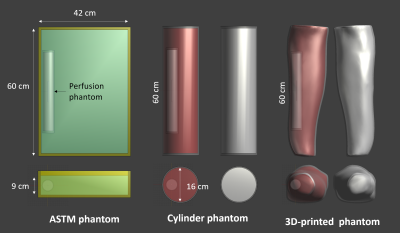
To address the geometry/tissue mimicking aspect, we have developed two muscle-mimicking phantom geometries (a simple cylinder, and an anatomically correct 3D-printed phantom) for evaluating heating in orthopedic IMDs. A muscle mimicking phantom material was adapted from Yuan et al3 for use at our frequencies of interest (64 & 128 MHz) and its’ electrical properties verified using the DAKS-12 (SPEAG, Zurich, Switzerland) connected to a Copper Mountain R60 (Copper Mountain, Indianapolis, IN, USA) and a PC running the DAKS software. Using the standard 10 cm Titanium rod from ASTM 2182-19a as our IMD, RF exposure in all three phantom geometries (ASTM, Cylinder, and 3D-printed) was simulated in Sim4Life (V6.2.0.4280, ZMT, Zurich, Switzerland) with both the standard gelled saline (Hydroxyethyl cellulose gel) and our muscle mimicking phantom. To address perfusion, a flow phantom was fabricated (Fig. 1) to mimic a perfused region of muscle, fed by a variable water pump set to the basal flow of muscle tissue (30 ml/min/kg). The perfusion phantom was simulated inside all three phantoms (Fig. 2), and Local SAR along the IMD was scaled to experimentally determined values of ~7.5 W/kg (64 MHz) and ~10.5 W/kg (128 MHz), which is typical of the titanium rod in the ASTM phantom. All FDTD simulations were run for 50 periods, global auto termination set to ‘Medium’, with 1 mm resolution subgridding around the perfusion phantom and titanium rod. Thermal simulations used the SAR distribution from the FDTD simulations to calculate implant heating using the Pennes Bioheat Equation. All thermal simulations were run using automatic gridding set to “Extremely Fine”, and titanium rod heating was simulated in each phantom first without the perfusion phantom, then with the perfusion phantom (but no perfusion), basal perfusion, and finally, full physiological perfusion.Results & Discussion
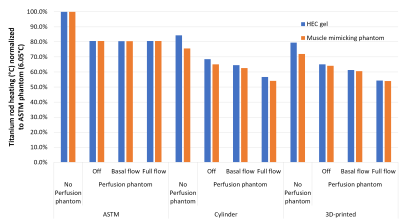
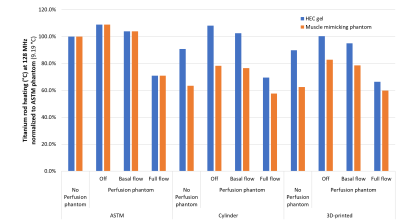
Across both frequencies and all phantom geometries/materials, full physiological perfusion lead to the most dramatic reduction in Ti-rod heating (up to 45%) compared to the other conditions, which was to be expected. At 64 MHz, both the cylindrical and 3D-printed phantom displayed lower Ti-rod heating compared to the ASTM phantom (with the greatest reduction seen in the fully perfused case).At 128 MHz, titanium rod heating was ~10% higher when placed inside the perfusion phantom with no flow, which could be explained by the sealed perfusion container being unable to dissipate heat to the rest of the phantom. Thus it seems as the phantom becomes more anatomically correct (both in terms of shape and electrical properties, and perfusion), there is a decrease in observed heating.Conclusion
These results suggest that the current gold standard (ASTM) phantom overestimates IMD heating, and true heating could be anywhere from 30-45% lower (dependent on perfusion capability and IMD exposure conditions). Full physiological perfusion is difficult to mimic experimentally due to the large temperature-dependent blood flow changes (up to 15 fold in muscle4), thus experimental validation (which is currently underway) will likely be based on static flow. Still, validating these simulations should allow a larger proportion of IMDs to pass the RF heating test, which should allow a larger proportion of patients currently living with IMD to continue getting access to MRI for years to come.Acknowledgements
The authors acknowledge financial support from NSERC and the Ontario Research Fund. The first author would also like to acknowledge Christopher Brown for his invaluable assistance with Sim4LifeReferences
1. ASTM F2182-19e2, Standard Test Method for Measurement of Radio Frequency Induced Heating On or Near Passive Implants During Magnetic Resonance Imaging, ASTM International, West Conshohocken, PA, 2019
2. ISO 10974. Assessment of the safety of magnetic resonance imaging for patients with an active implantable medical device, ISO, Switzerland, 2018.
3. Yuan, Yu, et al. "A heterogeneous human tissue mimicking phantom for RF heating and MRI thermal monitoring verification." Physics in Medicine & Biology 57.7 (2012): 2021.\
4. Murbach, Manuel, et al. "Thermal tissue damage model analyzed for different whole‐body SAR and scan durations for standard MR body coils." Magnetic resonance in medicine 71.1 (2014): 421-431.
Figures