2607
Isolating cardiac-related pulsatility in blood oxygenation level-dependent MRI with deep learning
Jake Valsamis1, Nicholas Luciw1, Nandinee Haq1, Sarah Atwi 1, Simon Duchesne 2,3, William Cameron1, and Bradley J MacIntosh1,4
1Physical Sciences, Sunnybrook Research Institute, Toronto, ON, Canada, 2Radiology Department, Faculty of Medicine, Laval University, Québec, QC, Canada, 3Quebec City Mental Health Institute, Québec, QC, Canada, 4Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada
1Physical Sciences, Sunnybrook Research Institute, Toronto, ON, Canada, 2Radiology Department, Faculty of Medicine, Laval University, Québec, QC, Canada, 3Quebec City Mental Health Institute, Québec, QC, Canada, 4Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada
Synopsis
Persistent exposure to highly pulsatile blood can damage the brain’s microvasculature. A convenient method for measuring cerebral pulsatility would allow investigation into its relationship with vascular dysfunction and cognitive decline. In this work, we propose a convolutional neural network (CNN) based deep learning solution to estimate cerebral pulsatility using only the frequency content from BOLD MRI scans. Various frequency component inputs were assessed, and echo time dependence was evaluated with a 5-fold cross-validation. Pulsatility was estimated from BOLD MRI data acquired on a different scanner to assess generalizability. The CNN reliably estimated pulsatility and was robust to various scan parameters.
Introduction
Vascular dysfunction is increasingly recognized as a key contributor to ageing, cognitive decline and dementia.1,2 One such dysfunction is the inability to dampen high-energy pulsatility from arteries to smaller vessels in the brain. Individuals with white matter hyperintensities tend to exhibit higher upstream pulsatility due to arterial stiffening.3 Chronic exposure to pulsatile blood can contribute to structural and functional changes in the brain.4Blood oxygenation level-dependent (BOLD) MRI is a form of image contrast that can yield whole-brain cardiac-related pulsatility information, provided that cardiac pulse recordings are synchronized with the MRI acquisition.5,6 Time-consuming image processing steps and the inconvenience of recording cardiac activity while scanning has prevented widespread adoption of this technique. In this work, we extend our deep learning efforts designed to isolate cardiac-related pulsatility from the BOLD data without the use of cardiac pulse information. We investigate the impact of input features and echo time on the performance of PulseMapper, the convolutional neural network (CNN) based deep learning architecture. Data from another vendor is used to preliminarily assess generalizability.
Methods
This study included 11 younger (ages 18-35) and 44 older (ages 55-85) cognitively healthy adult participants. Multi-echo BOLD data was collected on a Philips 3T Achieva scanner with TR=2300ms for TE1=13.82ms, TE2=35.35ms and TE3=56.89ms (2.88×2.88×4mm resolution). Cardiac pulse and respiratory motion information were recorded throughout the scan.Three cardiac-related pulsatility maps were generated per participant from the multi-echo BOLD scans and cardiac trace recordings to use as the ground truth for training the CNN. Respiration effects were removed from each BOLD scan using 3dretroicor.7 Images were skull stripped, slice time corrected, spatially smoothed (5mm at full width half maximum) and motion-corrected. BOLD volumes were resorted according to cardiac position. A seven-term Fourier series model was fit to the resorted data. Cardiac-related pulsatility maps were generated by calculating the root mean square (RMS) across the Fourier series beta coefficient terms.5
The proposed pipeline consisted of image feature extraction and pulsatility map generation (Figure 1A). First, a fast Fourier transform (FFT) was used to decompose each voxel’s BOLD signal into its frequency components. Next, these components served as input features to PulseMapper which consists of a U-net architecture with a pretrained ResNet-34 backbone on the descending arm.8,9 Training was done for 20 epochs with a learning rate of 0.001 and a batch size of 32. A weighted mean squared error loss function was used where brain voxels were given twice the weight of background voxels.
In the first experiment, designed to investigate the impact of input features, the dataset was divided into a training set (n=38) and a test set (n=17). Four input channel sizes were assessed by training four separate models with either 10, 20, 30, or 40 consecutive FFT features. Performance was evaluated by measuring the mean absolute error (MAE) between each voxel of the test set network estimated and ground truth pulsatility maps.
In a second experiment, a 5-fold cross-validation was used to investigate the dependence of network performance on echo time. 30 FFT features were used as input. The entire dataset was divided into five participant-level folds where 80% of the participants were used to train a new model and performance was evaluated with the remaining dataset.
A secondary single-echo BOLD dataset was collected on a Siemens 3T Prisma system (TR/TE=1550/30ms, 2.5mm isotropic resolution). This dataset was used to assess PulseMapper’s generalizability to data from a different vendor, acquired with different scanning parameters including spatial resolution, TR, and simultaneous multi-slice imaging.
Results
Network performance with varying input channel sizes is shown in Figure 2. The first 30 FFT feature maps resulted in the lowest test set MAE for all three echo times.A comparison between the network estimate and ground truth pulsatility maps, as well as the corresponding disparity map, is shown in Figure 3. Panels A and B demonstrate PulseMapper’s ability to predict contrast changes in the ground truth pulsatility map. Panel C shows images from a single-echo BOLD scan from another vendor.
The performance on the held-out test set from the cross-validation experiment is shown in Figure 4. Higher image similarity was observed for the images acquired at TE3=13.82ms.
Discussion
This study demonstrates that BOLD MRI and deep learning can be used to estimate cerebral pulsatility without relying on cardiac trace recordings. Using spectral features as inputs allows PulseMapper to effectively characterize periodic cardiac signals. Figures 3A and B show PulseMapper’s tendency to overestimate pulsatility in regions with high ground truth pulsatility and underestimate pulsatility elsewhere. High disparity in Figure 3C shows globally overestimated pulsatility on the Siemens test data; nevertheless, these estimates are promising because training was performed with data from a different scanner with different temporal and spatial resolutions. The 5-fold cross-validation shows a general trend of improved performance with increasing TE, however, the overlapping interquartile ranges suggest that PulseMapper is not heavily dependent on TE. Future work will involve training PulseMapper on multi-vendor data and examining pulsatility predictions in different patient populations.Acknowledgements
This research was supported by a CIHR project grant and an industry-partnership INOVAIT grant.References
- Gorelick P, Scuteri A, Black E, DeCarli C, Greenberg S, and Lopez O, “Vascular Contributions to Cognitive Impairment and Dementia,” Stroke, vol. 42, no. 9, pp. 2672–2713, 2011.
- Wardlaw J et al., “Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration,” Lancet Neurol., vol. 12, no. 8, pp. 822–838, 2013.
- Webb A, Simoni M, Mazzucco A, Kuker W, Schulz U, and Rothwell P, “Increased cerebral arterial pulsatility in patients with leukoaraiosis: arterial stiffness enhances transmission of aortic pulsatility,” Stroke, vol. 43, no. 10, pp. 2631–6, 2012.
- Kim T et al., “Cardiac-induced cerebral pulsatility, brain structure, and cognition in middle and older-aged adults,” NeuroImage, vol. 233, 2021.
- Atwi S et al., “Cardiac-Related Pulsatility in the Insula Is Directly Associated With Middle Cerebral Artery Pulsatility Index,” J. Magn. Reson. Imaging, vol. 51, no. 5, pp. 1454–1462, 2020.
- Theyers A, Goldstein B, Metcalfe A, Robertson A, and MacIntosh B, “Cerebrovascular blood oxygenation level dependent pulsatility at baseline and following acute exercise among healthy adolescents,” J. Cereb. Blood Flow Metab., vol. 39, no. 9, pp. 1737–1749, 2019.
- Glover G, Li T, and D Ress, “Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR,” Magn. Reson. Med., vol. 44, no. 1, pp. 162–167, 2000.
- Ronneberger O, Fischer P, and Brox T, “U-Net: Convolutional Networks for Biomedical Image Segmentation,” Med. Image Comput. Comput.-Assist. Interv., vol. 3, pp. 234–241, 2015.
- He K, Zhang X, Ren S, and Sun J, “Deep Residual Learning for Image Recognition,” Proc. IEEE Conf. Comput. Vis. Pattern Recognit., pp. 770–778, 2016.
Figures
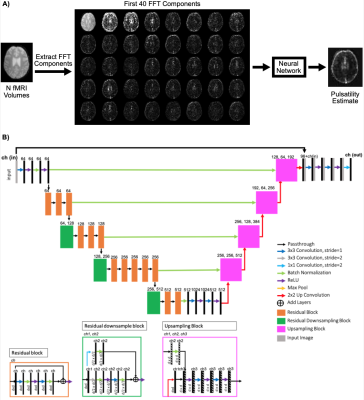
Figure 1. A) Image processing pipeline. B) Network architecture. In each residual and upsampling block, d is the dimension of the image.
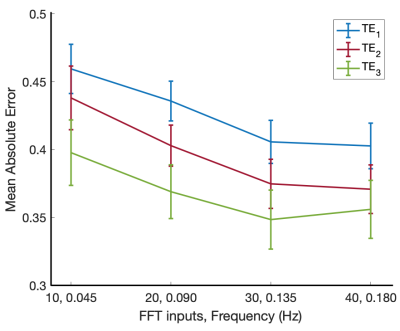
Figure 2. Mean absolute error between the ground truth pulsatility maps and the network estimated maps as the number of FFT inputs is increased. Performance was evaluated on the held-out test set.
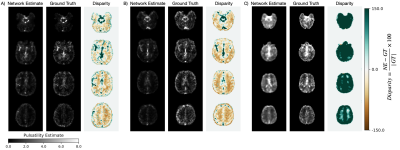
Figure 3. Network estimated (NE) pulsatility maps alongside the ground truth (GT) and disparity images. Panel A) and panel B) show the individuals with mean and highest MAE, respectively, from the TE2 held-out test set. Panel C) shows a preliminary attempt to assess generalizability with network estimate and ground truth images from single-echo BOLD data from another vendor, acquired with different scanning parameters including spatial resolution, TR, and simultaneous multi-slice imaging.
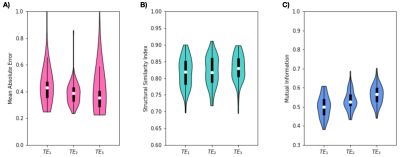
Figure 4: Five-fold cross-validation performance of the network for different echo times using 30 FFT components as input features. (A) Mean absolute error, (B) Structural Similarity Index and (C) Mutual information from the held-out test set. Results from all the participants are shown by virtue of the five-fold cross-validation.
DOI: https://doi.org/10.58530/2022/2607