2598
Mitigation of Magnetisation Transfer Effects using Off-Resonance Pulses in MR Fingerprinting1Surgery and Cancer, Imperial College London, London, United Kingdom, 2Radiotherapy and Imaging, Institute of Cancer Research, LONDON, United Kingdom, 3Research and Collaborations GB&I, Siemens Healthcare Ltd, Frimley, United Kingdom, 4London Institute of Medical Sciences,Medical Research Council, Imperial College London, London, United Kingdom, 5National Heart and Lung Institute, Imperial College London, London, United Kingdom, 6Brain Sciences, Imperial College London, London, United Kingdom, 7Department of Bioengineering, Imperial College London, London, United Kingdom, 8Department of Imaging, Imperial College Healthcare NHS Trust, London, United Kingdom, 9Department of Radiotherapy and Imaging, Institute of Cancer Research, London, United Kingdom, 10Department of Radiology, Royal Marsden Hospital, London, United Kingdom, 11Radiotherapy and Imaging, Institute of Cancer Research, London, United Kingdom, 12Department of Medical Physics, Royal Surrey NHS Trust, London, United Kingdom
Synopsis
Previous work has shown an inherent bias in quantitative T1 values for current standard MRF techniques as compared to gold standard methods. In our study we show how magnetisation transfer effects are a component of this bias and that the introduction of off-resonance pulses within an MRF sequence may mitigate these effects.
Introduction
In conventional T1 and T2 weighted MR images, the absolute signal intensity depends on multiple relaxation mechanisms and cannot be used quantitatively for diagnosis. Quantitative relaxometry could improve tissue classification and clinical assessment of pathology in the brain. However, long acquisition times mean quantitative relaxometry is currently clinically infeasible.1 Magnetic Resonance Fingerprinting (MRF) is a rapid quantitative imaging framework. A prototype fingerprinting sequence has previously been shown to give T1 values consistently lower than Variable Flip Angle (VFA) mapping and T2 values consistently lower than Multi Echo Spin Echo (MESE) mapping in brains of healthy volunteers 2 and brain tumour patients 3, especially in white matter regions.In this study we investigate whether this bias is due to Magnetisation Transfer (MT) taking place, and if it can be mitigated by the inclusion of off-resonance pulses. It is hypothesised that off-resonance pulses will saturate the bound pool and suppress signal from areas with significant bound and free pool interactions, hence T1 and T2 maps reconstructed from the data acquired with off-resonance pulses should have T1 and T2 values closer to ground truth due to mitigated MT dependence when compared with quantitative maps acquired without off-resonance pulses.
Methods
A FISP 2D cartesian MRF sequence with and without off resonance pulses before every readout was developed in house with varying flip angles and repetition times based on schedules reported by Jiang et al 4 . A series of FISP acquisitions were acquired with a sinusoidal variation of flip angles (FA) and repetition times (TR) in a Perlin noise pattern. The FOV was 256 and the acquisition matrix was 128 x 126. 64 lines of k space per image were acquired with GRAPPA-2 acceleration.Initial T1 investigations in a single healthy volunteer were carried out as part of an ethically approved study after obtaining informed consent. A total of 409 contrasts acquired with a slice thickness of 6.5 mm to reconstruct a time-series of complex images with a resolution of 2mm x 2mm. T2 estimates were not investigated as part of this study. The off-resonance pulse was 180° with a frequency offset of 2000 Hz. Data acquisition with and without the off-resonance pulses was performed on a Siemens MAGNETOM Prisma 3 Tesla system (Siemens Healthineers, Erlangen Germany).
A dictionary was generated using a single-pool extended phase graph (EPG) model developed by Malik et al 5 for T1 values 10-4500 ms and T2 values 2-3000 ms. MT effects were not explicitly encoded in the dictionary. Fingerprints for every voxel were extracted and matched to the closest dictionary entry using a maximum dot product search, and corresponding T1 and T2 maps were reconstructed. Proton density maps were reconstructed from the scale of each voxel evolution. Regions of interest were drawn manually in areas of white and grey matter for comparison of T1 maps.
Results
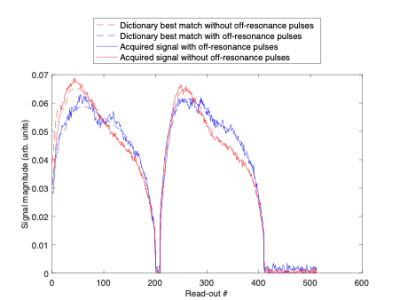
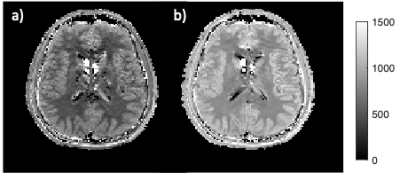
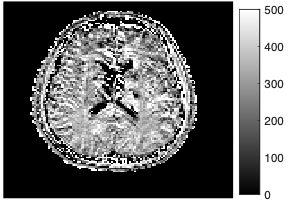
In Figure 1, signal evolutions for a single white matter voxel acquired with and without off resonance pulses are displayed together with their corresponding best matching dictionary entries. Fingerprints with off-resonance pulses had a lower amplitude due to regions in the free pool exchanging with the bound pool becoming saturated and therefore producing a lower measured signal. Differences between the signal evolutions with and without off-resonance pulses (Figure 1) resulted in a different best matching dictionary entries (Figure 2). From ROI-averaged analysis, the T1 estimate in white matter increased by 69.7% (from 479 ms to 814 ms), and by 40% (from 750 ms to 1056 ms) in grey matter.Discussion
T1 values obtained using an MRF acquisition scheme were found to increase in grey and white matter when off-resonance pulses were integrated into the pulse sequence. The effect was greater in white matter, as compared to grey matter. This is to be expected as MT is correlated with axonal count and myelin content, which are greater in white matter 6. In a study by Hilbert et al 7, longer T1s were observed in white matter when MT effects were explicitly encoded when simulating the dictionary. Magnetisation transfer effects have been shown to affect quantification of T1 and T2 and can be mitigated in steady-state approaches pulse sequences that transmit a constant RF power 8. Numerical derivations by Texeria et al 8 imply a constant saturation of the bound pool should yield behaviour similar to a single-pool in a transient experiment such as MRF. Our results suggest 180° MT pulses with a frequency offset of 2000Hz applied every TR may be sufficient to achieve these conditions.Conclusion
MT effects are a component of the bias observed in current standard MRF techniques as compared to gold standard methods. We have shown that the introduction of off-resonance pulses within an MRF sequence may address MT effects observed. Further work is needed to assess resultant quantitative T2 images.Acknowledgements
The authors would like to thank: the volunteers who participated in the study, Shaihan Malik for assistance with the EPG formalism, and the Imperial MRI Physics Collective. This study was funded by the Imperial-ICR Cancer Research UK Convergence Science Centre and the Medical Research Council, MGS was part funded by the National Physical Laboratory’s Industrial Strategy Challenge Fund (ISCF) Medical Imaging Accelerator programme financed by the Department for Business, Energy and Industrial Strategy’s ISCF.References
1. Cheng, M. et al. Practical medical applications of quantitative MR relaxometry. Journal of Magnetic Resonance Imaging vol. 36 805–824 (2012).
2. Smith, J. et al. Validity and reproducibility of Magnetic Resonance Fingerprinting in the healthy human brain at 3T. in Proceedings of the 28th Annual Meeting of the International Society of Magnetic Resonance in Medicine (2020).
3. Kukran, S. et al. Validity and repeatability of MRF in glioma and normal appearing contralateral brain tissue at 3T. in Proceedings of the 29th Annual Meeting of the International Society of Magnetic Resonance in Medicine (2021).
4. Jiang, Y., Ma, D., Seiberlich, N., Gulani, V. & Griswold, M. A. MR fingerprinting using fast imaging with steady state precession (FISP) with spiral readout. Magn. Reson. Med. 74, 1621–1631 (2015).
5. Malik, S. J., Teixeira, R. P. A. G. & Hajnal, J. V. Extended phase graph formalism for systems with magnetization transfer and exchange. Magn. Reson. Med. 80, 767–779 (2018).
6. Schmierer, K., Scaravilli, F., Altmann, D. R., Barker, G. J. & Miller, D. H. Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Ann. Neurol. 56, 407–415 (2004).
7. Hilbert, T. et al. Magnetization transfer in magnetic resonance fingerprinting. Magn. Reson. Med. 84, 128–141 (2020).
8. Rui, R. P. et al. Controlled saturation magnetization transfer for reproducible multivendor variable flip angle T 1 and T 2 mapping. Magn. Reson. Med. 84, 221–236 (2020).
Figures