2593
Towards A Clinical Prostate MR Fingerprinting Protocol1Philips Research Europe, Hamburg, Germany
Synopsis
We present an efficient MR Fingerprinting (MRF) protocol for prostate imaging at 3T. Full prostate coverage is achieved in 04:20 min with no considerable reconstruction latency. Dixon-MRF has been implemented in the scanner software and combined with a B1+ DREAM scan in a fully-integrated B1-corrected MRF reconstruction. Furthermore, flow compensation is included to suppress artifacts caused by blood flow in large vessels. The proposed prostate MRF protocol has been evaluated in six healthy volunteers.
1 Introduction
We present an efficient spiral MR Fingerprinting1 protocol for quantitative imaging of the prostate at 3T in less than five minutes. Compared to previously presented prostate MRF protocols2,3 we use an additional chemical shift based water/fat separation (Dixon-MRF), and perform a fully-integrated DREAM pre-scan for B1+ correction in MRF. The fast reconstruction using the scanner’s built-in GPU allow MRF parameter maps to be reconstructed on the console without disturbing the clinical workflow.2 Methods
2.1 MRF acquisition & reconstructionAn MRF protocol of 15 slices (slice thickness 5 mm), FoV of 200 x 200 mm2 with 80 mm oversampling (in-plane resolution of 1.1 mm) has been designed for full coverage of the prostate. A flip angle train of length 1000 with interleaved echo times4 and TR = 17 ms is used, cf. Figure 1. For each flip angle, a single-spiral with undersampling of 29 was aquired. Joint spiral deblurring and water/fat separation5 is performed on each data tuple along the MRF train. The resulting water-only (fat-suppressed) MRF images are matched against a dictionary with T1 = [0, 3000] ms, T2 = [0, 1000] ms (both discretized on a log-scale of 2% step size), and B1+ = [0.75, 1.25] with at linear step size of 1%. The MRF reconstruction was integrated in the scanner software using a GPU backend that resulted in a latency of less than ten seconds for the multi-slice acquisition.
Additionally, we apply flow compensation where an extra gradient in slice selection direction is added such that its first moment vanishes and, thus, flow of constant through-plane velocity is expected to cancel out.
2.2 Protocol details
Our protocol consists of the following fully-integrated steps:
(i) A volumetric DREAM6 pre-scan (similar to a B0 pre-scan performed for, e.g., spiral deblurring) for B1+ measurement on a coarser and larger geometry compared to (ii) is acquired in a multi 2D acquisition (FoV = 470 x 470 mm2, slices = 20, slice thickness = 10 mm, pixel size = 5.88 mm, TR = 4.1 ms, averages = 4, Uniformity = ’CLEAR’, STEAM angle = 80, total scan time = 01:07 min). An RF Shim volume is placed in the area of the prostate in order to achieve a homogeneous map ∼ 100% in that body region.
(ii) Multi 2D MR-Fingerprinting as described in Subsection 2.1 with joint spiral deblurring and water/fat separation according to Wang5. The map obtained in (i) is automatically interpolated onto the present geometry, and is used for corrected dictionary matching.
The suggested prostate MRF protocol consists of the scans (i) and (ii), and a typical output for the fat-suppressed case is shown in Figure 2. T1 and T2 maps of the prostate of six healthy volunteers (informed consent obtained) have been obtained on a 3T MRI system (Ingenia, Philips Healthcare). Additionally, a 2D multi-echo spin-echo scan for T2 comparison was performed:
(iii) 2D multi-echo spin-echo (MESE) scan located at a relevant slice obtained in (ii) for T2 reference. Parameters: TR = 5 s, 14 echoes, TEn = n*16 ms, 160$$$^\circ$$$ constant refocusing, acquisition voxel 0.96 x 0.96 x 3 mm3, oversampling 135 mm, Compressed SENSE R=7, total single slice scan time = 06:00 min.
3 Results and Discussion
The presented prostate Dixon-MRF protocol has been optimized with respect to overall scan time and resolution. In Figure 3, we show a comparison between MRF with and without flow compensation for a single volunteer. We note strong spiral artifacts that cause rings centered around both arteries affecting the T1 values in the prostate. Flow compensation leads to a much smaller and more local effect of the blood flow and, thus, corrects the values of T1/T2 in the prostate. B1+ correction was performed in all cases. The placement of the RF Shim volume yields values near 100% in the prostate with larger variations in other body parts, cf. Figure 2(a).Figure 4 shows T1, T2 MRF and the reference MESE T2 map for all considered volunteers. A good agreement of the respective T2 distribution is observed. The discrepancy in T2 magnitudes comparing water/fat resolved MRF and the MESE reference in Figure 4 may be explained by a combination of stimulated echo effects (prolonging T2 results in MESE7) and magnetization transfer effects, prolonging T2-MESE and shortening T2 results in MRF, because they are not considered for the dictionary (this would require a 2-pool model8).
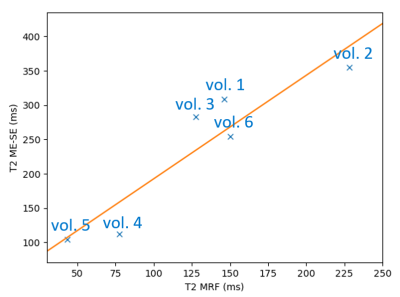
Figure 5 shows the MRF T2 values evaluated in the normal-appearing peripheral zone (NPZ) for all six volunteers. We note a strong variation in T2 in the considered ROI over all volunteers but observe a nearly linear behaviour when comparing to the MESE values.
4 Conclusion
A high-quality, high SNR, prostate MRF protocol with additional B1+ correction from a fully-integrated DREAM scan (+1 min) in less than 5 mins is presented. The extension by a robust water/fat separation can be used especially in regions where fat-induced blurring affects the image quality. We further provided an instantaneous reconstruction workflow that makes the new protocol attractive in clinical practice. A comparison with (standard) MESE T2 mapping (single-slice acquisition in 6 mins) shows good correlation with the maps obtained from MRF and give confidence for evaluation in further clinical studies.Acknowledgements
We would like to thank Victoria Yu, Can Wu, Ouri Cohen, Ergys Subashi, and Ricardo Otazo from MSKCC for helpful discussions on the topic.References
(1) Ma, D.; Gulani, V.; Seiberlich, N.; Liu, K.; Sunshine, J. L.; Duerk, J. L.; Griswold, M. A. Nature 2013, 495, 187–192, DOI:10.1038/nature11971.
(2) Yu, A. C.; Badve, C.; Ponsky, L. E.; Pahwa, S.; Dastmalchian, S.; Rogers, M.; Jiang, Y.; Margevicius, S.;Schluchter, M.; Tabayoyong, W.; Abouassaly, R.; McGivney, D.; Griswold, M. A.; Gulani, V. Radiology 2017, 283, 729–738, DOI:10.1148/radiol.2017161599.
(3) Shiradkar, R. et al. European Radiology 2021, 31, 1336–1346, DOI:10.1007/s00330-020-07214-9.
(4) Koolstra, K.; Webb, A. G.; Veeger, T. T. J.; Kan, H. E.; Koken, P.; Börnert, P. Magnetic Resonance in Medicine 2019, 84, 646–662, DOI:10.1002/mrm.28143.
(5) Wang, D.; Zwart, N. R.; Pipe, J. G. Magnetic Resonance in Medicine 2018, 79, 3218–3228, DOI:10.1002/mrm.26950.
(6) Nehrke, K.; Börnert, P. Magnetic Resonance in Medicine 2012, 68, 1517–1526, DOI:10.1002/mrm.24158.
(7) Maier, C. F.; Tan, S. G.; Hariharan, H.; Potter, H. G. Journal of Magnetic Resonance Imaging 2003, 17, 358–364, DOI:10.1002/jmri.10263.
(8) Hilbert, T.; Xia, D.; Block, K. T.; Yu, Z.; Lattanzi, R.; Sodickson, D. K.; Kober, T.; Cloos, M. A. Magnetic Resonance in Medicine 2019, 84, 128–141, DOI:10.1002/mrm.28096.
Figures