2590
Preliminary development of a Magnetic Resonance Fingerprinting framework for fast multiparametric low-field NMR relaxometry1Department of Physics and Astronomy "Augusto Righi", University of Bologna, Bologna, Italy, 2INFN, Istituto Nazionale di Fisica Nucleare, Sezione di Bologna, Bologna, Italy, 3Department of Radiology, Stanford University, Stanford, CA, United States, 4Department of Mathematics, University of Bologna, Bologna, Italy, 5Department of Civil, Chemical, Environmental, and Materials Engineering, University of Bologna, Bologna, Italy
Synopsis
A novel application of Magnetic Resonance Fingerprinting (MRF) on low-field NMR is presented. To successfully implement MRF, the correlation between the static and the radio-frequency fields has to be measured, because the evolution of the signal cannot be analyzed without accounting for magnetic fields inhomogeneities. Experimental results have been validated by simulations and compared using the RMSE. This preparatory evaluation allows the use of MRF for NMR parameters quantification. Then, an artificial intelligence approach for parameters reconstruction has been used to overcome the limitation of the standard dictionary approaches when several parameters have to be estimated.
Introduction
NMR relaxometry based on compact low-field spectrometers has a variety of applications ranging from quality testing in the pharmaceutical1 and food2 industries to biomedicine and pre-clinical research3-5. Multidimensional methodologies enable correlated multiparametric information, such as T1-T2 maps, to be obtained. However, the long scan time required from multidimensional pulse sequences (order of tens of minutes) challenges and limits their applicability when biological samples are investigated.Magnetic Resonance Fingerprinting (MRF) is an MRI methodology that allows multiparametric mapping in a one-shot measurement6 exploiting a pulse sequence designed to make the magnetization evolution simultaneously dependent from multi MR parameters (the so-called fingerprint). A pattern recognition method is used to match the experimental signal with a dictionary of simulated fingerprints.
Translating the MRF paradigm to low-field NMR may enhance the use of NMR relaxometry for biomedical application, allowing ultra-fast multiparametric characterization. However, the application of MRF to compact low-field devices is challenged by static (B0) and excitation (B1) field inhomogeneities, which are more severe than those found in high-field MRI scanners. In this preliminary study, we investigate the feasibility of developing a MRF framework with a low-field NMR device.
Methods
Two samples, rubber and CuEDTA-doped distilled water were scanned with a low-field NMR customized Jeol C-60 electromagnet (B0=20MHz) using the KEAII (Magritek, NZ) spectrometer.The acquisition protocol included a 2D-ANGLE-FID sequence for B0-B1 characterization7, a novel IR-bSSFP-like sequence to acquire the MRF signal (96 pulses with flip angle varying within the [5°-70°] range at fixed TR=1ms, see Fig.1), and standard CPMG and IR sequences for ground-truth T1 and T2 characterization.
The 2D-ANGLE-FID acquired multiple Free-Induction-Decays (FID) (tacq=10ms, sampling rate:12.5µs) varying the excitation pulse lengths within the range [0.5-100] µs with a 2µs step increments (see Fig.1). A 2D FFT algorithm was used to reconstruct the B0-B1 correlation map (See Fig.2). B1 was expressed as efficiency ratio of the measured B1 value to the nominal one (B1nominal=1mT).
The reference T1 and T2 values of the rubber sample were used to verify the ability to simulate the real MRF signals (Bloch simulations using in-house Matlab toolbox, MARSS8) using both the mean (B0, B1) values within the sensitive volume, as done for the MRI methodology, and using the whole B0-B1 map. The root-mean-squared-error between real and simulated MRF signals were used as similarity metric.
The full MRF framework (acquisition + parameter estimation) was tested by training a fully connected Deep Neural Network9 (fc-NN) with 100000 simulated MRF signals generated randomly sampling (T1, T2) pairs with T1ϵ[1, 4000] ms and T2ϵ[1, 2000] ms (Fig.3).
The pre-trained fc-NN was used to predict the CuEDTA-doped water (T1,T2) values and compared with the reference values.
Results
The correlation between B0 offset and B1 efficiency in the sensitive volume of the low-field NMR device acquired with the rubber sample is shown in Fig.2. The magnetic fields were characterized by bimodal distributions and their correlation showed a highest peak centered at B0 = -150 Hz and B1 = 1.4.Main results of synthetic MR Fingerprints generated by using T1 and T2 values of the rubber sample by standard sequences are represented in Fig. 4. The simulation was in good agreement (RMSE=6.6·10-3) with the experimental signal when accounting for the B0-B1 map (Fig.4 on the right), but not (RMSE=1.8·10-2) when mean B0 and B1 values were considered (Fig.4 on the left).
Simulated and acquired MRF signals of the CuEDTA sample are shown in Fig. 5. The synthetic signal was generated using the T1 and T2 values predicted by the fc-NN algorithm. A good match was obtained (RMSE =3·10-4). The predicted T1 and T2 were 3.2 ms and 2.1 ms respectively, compared to the reference T1=2.2 ms, and T2=1.9 ms, measured by IR and CPMG.
Discussion
This preliminary study showed the feasibility of applying the MRF framework using low-field instruments since the simulated MRF signal and the experimental MRF data were in excellent agreement. This was achieved using an accurate characterization of the B0-B1 correlation map. Quantitative estimation of T1 and T2 was possible and accurate as demonstrated by the comparison with the gold standard quantitative techniques.A limitation of the current framework is the need to obtain the B0-B1 map which is indispensable to accurately simulate the MRF signal but increases the scan time. Future studies based on machine learning techniques should be investigate how to eliminate the need of acquiring an auxiliary 2D ANGLE-FID sequence.
Although our framework needs further testing on samples characterized by different relaxation rates, these promising results highlight the feasibility to extend the MRF framework towards biomedicine application using low-field NMR devices where fast acquisition is required, such as in-vitro analysis of biological tissues whose characteristics may change within the acquisition time required by standard sequences.
Conclusion
MRF can be applied in the framework of low-field NMR apparatus and can give robust quantitative evaluation of T1 and T2 with an acquisition time on the order of tens of seconds which is more than 10 times lower than standard experiments for T1 and T2 quantification (CPMG and IR acquisition times are on the order of tens of minutes).Acknowledgements
We received financial support from the GIDRM association10: 2021 Segre-Capitani grant.
References
1. Schumacher SU, Rothenhäusler B, Willmann A, Thun J, Moog R, Kuentz M. Time domain NMR as a new process monitoring method for characterization of pharmaceutical hydrates. J. Pharm. Biomed. Anal. 2017;137:96–103 doi: 10.1016/j.jpba.2017.01.017.
2. Lin X, Ruan R, Chen P, et al. NMR state diagram concept. J. Food Sci. 2006;71 doi: 10.1111/J.1750-3841.2006.00193.X/FORMAT/PDF.
3. Navon G, Eliav U, Demco DE, Blümich B. Study of order and dynamic processes in tendon by NMR and MRI. J. Magn. Reson. Imaging 2007;25:362–380 doi: 10.1002/jmri.20856.
4. Ali TS, Tourell MC, Hugo HJ, et al. Transverse relaxation-based assessment of mammographic density and breast tissue composition by single-sided portable NMR. Magn. Reson. Med. 2019;82:1199–1213 doi: 10.1002/mrm.27781.
5. Barbieri M, Fantazzini P, Bortolotti V, et al. Single-sided NMR to estimate morphological parameters of the trabecular bone structure. Magn. Reson. Med. 2021;85:3353–3369 doi: 10.1002/MRM.28648/FORMAT/PDF.
6. Ma D, Gulani V, Seiberlich N, et al. Magnetic resonance fingerprinting. Nature 2013;495:187–92 doi: 10.1038/nature11971.
7. Topgaard D, Sakellariou D, Pines A. NMR spectroscopy in inhomogeneous B 0 and B 1 fields with non-linear correlation. 2005 doi: 10.1016/j.jmr.2005.03.006.
8. Barbieri M, Brizi L, Bortolotti V, Fantazzini P, Sykora S, Testa C. A flexible software for NMR pulse sequence simulations, first applications to MR Fingerprinting: Quantification of myelin water fraction in white matter and diffusion coefficient measurement. In: ISMRM workshop on Magnetic Resonance Fingerprinting. Cleveland; 2017.
9. Barbieri M, Brizi L, Giampieri E, et al. A deep learning approach for magnetic resonance fingerprinting: Scaling capabilities and good training practices investigated by simulations. Phys. Medica 2021;89:1120–1797 doi: 10.1016/j.ejmp.2021.07.013.
10. The Italian Group of Magnetic Resonance, url: http://www.gidrm.org
Figures
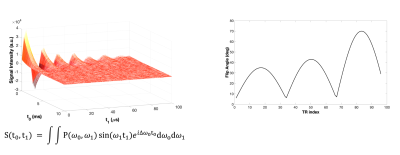
On the left, the signal acquired from an ANGLE-FID sequence for a sample of rubber with relaxation times T1 = 51.7 ms, T2 = 2.59 ms. On the right, the Flip Angle pattern used for the IR-BSSFP-like sequence.
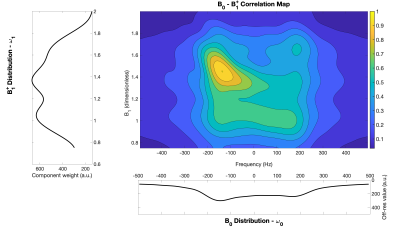
Correlation map between magnetic fields for a sample of rubber with the single distribution of each fields.
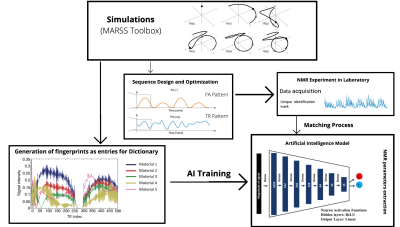
Framework of proposed method.
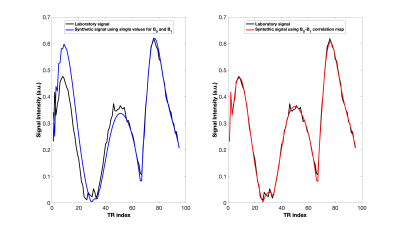
Comparison between experimental signal and simulated one for the case of Rubber sample with T1=51.7 ms, and T2=2.59 ms. On the left, the synthetic signal obtained considering one value for B0 and B1 (B0 = -150 Hz and B1 = 1.4). The RMSE value obtained was 1.8·10-2. On the right, the synthetic signal obtained considering the B0-B1 correlation map. The RMSE value obtained was 6.6·10-3.
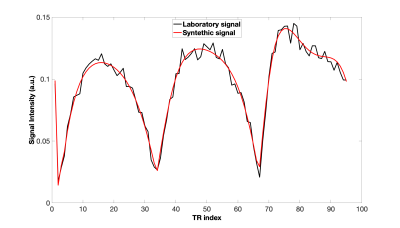
Comparison between experimental signal and simulated one for the case of CuEDTA sample with T1 = 2.2 ms, and T2 = 1.9 ms. The relaxation times extracted by the Network were T1 = 3.2 ms, and T2 = 2.1 ms. The RMSE value obtained was 3·10-4.