2583
Tumor stiffness based on 3D MR Elastography as a marker for predicting the aggressiveness in cervical cancer1Department of Radiology, The third affiliated hospital of Sun Yat-sen University, Guangzhou, China, 2Department of Radiology, Mayo Clinic College of Medicine, Mayo Clinic, Rochester, MN, United States
Synopsis
Preoperative prediction of aggressiveness (histologic subtype, grade of differentiation, Federation International of Gynecology and Obstetrics (FIGO) stage) in cervical cancer (CC) using a noninvasive method remains a challenge. 3D MR Elastography (MRE) is a novel functional MRI technique which can quantitatively characterize the mechanical properties of tumors. We retrospectively analyzed 24 CC patients undergoing 3D MRE examinations and found that tumor stiffness (TS) in adenocarcinoma and FIGO stage III/IV was significantly higher than that in squamous cell carcinoma and FIGO stage I/II. TS based on 3D MRE could serve as a potential noninvasive marker to assess the aggressiveness in CC.
Introduction
Cervical cancer (CC) is the third most common malignancy worldwide, which has been the second leading cause of cancer deaths among women [1]. Tumor’s aggressiveness is closely associated with risk of the lymph node metastases, distant relapse and recurrence in CC patients [1-3]. Preoperative evaluation of aggressiveness for patients with CC is of great significance to choose the optimal clinical treatment. MR elastography (MRE) has high diagnostic performance in detecting and staging liver fibrosis[4-6]. Previous studies have suggested MRE could be applied in identifying benign from malignant tumors, predicting tumor grade and prognosis[7-11]. As for MRE applied in the uterus, only one study was involved in malignant uterine tissue and they proposed that 3D MRE may be useful in the preoperative risk stratification of endometrial carcinoma [12]. However, the value of 3D MRE in assessing the aggressiveness of CC has not been explored. The purpose of this study was to investigate whether TS based on 3D MRE could preoperatively predict the aggressiveness of CC.Methods
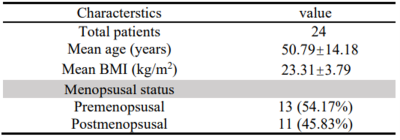
Following ethics committee approval with a waived informed consent requirement, 24 histopathology-proven CC patients with 60 Hz MRE examinations were enrolled retrospectively from August 2015 to January 2018. The specific parameters of 3D EPI MRE sequence are as follows: TR 1650ms, TE 56.3 ms, FOV 24cm×24 cm, matrix 80×80, Layer thickness 3mm, layer spacing 1mm, bandwidth 250kHZ, acceleration factor 2, NEX 2, MEGs frequency 155Hz, cyclic motion coding gradient direction 32, exciter amplitude 100%, continuous vibration mode, shear wave frequency is 60Hz.Patients were divided into two subgroups according to the pathological type (squamous cell carcinoma vs. adenocarcinoma group, 20 vs. 4), degree of differentiation (well/moderately vs. poorly differentiated group, 20 vs. 4) and FIGO stage (FIGO I/II vs. III/IV group, 16 vs. 8). Regions of interest (ROIs) were carried out in the whole tumor and drawn by two experienced radiologists (>5 years of experience in pelvic MRI), and the mean TS was calculated finally. The ROIs excluded tumor edges, areas of significant wave interference and any other artifacts seen in the magnitude and wave images, with reference to conventional MR images. Independent sample T test or Mann-Whitney U nonparametric test was used to compare stiffness between different groups. Receiver operating characteristic (ROC) curve analyses were performed to assess the performance of 3D MRE in predicting the aggressiveness of CC. Statistical significance was defined as P<0.05.Results
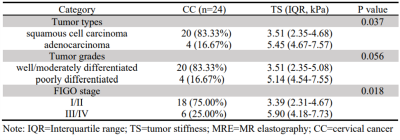
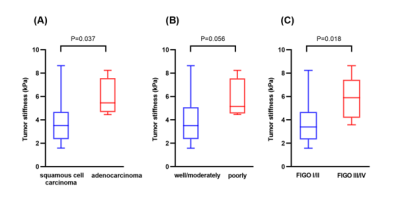
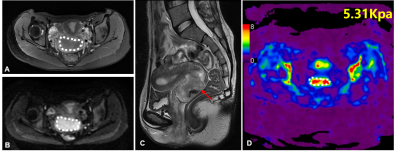
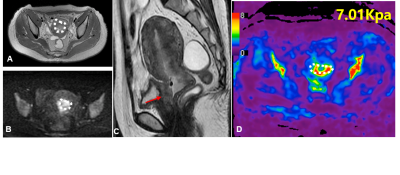
Clinical information about the enrolled patients is summarized in Table 1. TS in adenocarcinoma group was significantly higher than that in squamous cell carcinoma group (5.45 kPa vs. 3.51 kPa, P=0.037). The cutoff value of TS to discriminate adenocarcinoma from squamous cell carcinoma was 4.42 kPa with a sensitivity of 100%, specificity of 70.0%, and ROC curve area of 0.84 (95% CI: 0.68-1.00). TS in poorly differentiated CC was higher than that in well/moderately differentiated CC (5.14 kPa vs. 3.51 kPa), but the difference was not significant (p=0.056). TS of FIGO stage III/IV CC was significantly higher than that of FIGO stage I/II CC (5.90 kPa vs. 3.39 kPa, P=0.018). The cutoff value of TS to discriminate FIGO stage I/II CC from FIGO stage III/IV EC was determined at 4.24 kPa, with a sensitivity of 83.3%, specificity of 72.2%, and ROC curve area of 0.82 (95% CI: 0.65-1.00). Detailed information about the different groups of CC is described in Table 2, and the corresponding box plot is showed in Figure 1. Representative cases are exhibited in Figures2,3.Discussion
To our knowledge, this is the first study focusing on the value of 3D MRE for predicting the aggressiveness of CC. We found that TS quantified by 3D MRE has potential for predicting histologic subtype and FIGO stage of CC. More aggressive tumors characteristically exhibited highly invasive properties, such as rapid proliferation and the rich blood supply, which leaded to the high cellularity and the increased tumor interstitial fluid pressure, eventually resulting in the increase TS [12,13]. Compared to well/moderately differentiated CC, TS in poorly differentiated CC was higher but the difference between them wasn’t significantly, which may be explained by the limited numbers of the poorly differentiated CC. This was a small sample exploratory study in a single-center. A multicenter study in a larger population would be needed to validate our findings in the future.Conclusion
This study provides preliminary evidence that MRE-assessed TS shows promise as a biomarker in clinical practice to assess the aggressiveness of CC preoperatively.Acknowledgements
The authors state that this study has received funding by the National Natural Science Foundation of China grant 91959118 (JW), Clinical Research Foundation of the 3rd Affiliated Hospital of Sun Yat-Sen University YHJH201901 (JW), and Guangdong Basic and Applied Basic Research Foundation (No.2021A1515010582) (JW).References
1. Johnson CA, James D, Marzan A, Armaos M (2019) Cervical cancer: An overview of pathophysiology and management. Semin Oncol Nurs 35:166-174
2. Bourgioti C, Chatoupis K, Moulopoulos LA (2016) Current imaging strategies for the evaluation of uterine cervical cancer. World J Radiol 8:342-354
3. Haldorsen IS, Lura N, Blaakaer J, Fischerova D, Werner H (2019) What is the role of imaging at primary diagnostic Work-Up in uterine cervical cancer? Curr Oncol Rep 21:77
4. Idilman IS, Li J, Yin M, Venkatesh SK (2020) MR elastography of liver: Current status and future perspectives. Abdom Radiol (NY) 45:3444-3462
5. Morisaka H, Motosugi U, Ichikawa S et al (2018) Magnetic resonance elastography is as accurate as liver biopsy for liver fibrosis staging. J Magn Reson Imaging 47:1268-1275
6. Pepin KM, Ehman RL, McGee KP (2015) Magnetic resonance elastography (MRE) in cancer: Technique, analysis, and applications. Prog Nucl Magn Reson Spectrosc 90-91:32-48
7. Li M, Guo J, Hu P et al (2021) Tomoelastography based on multifrequency MR elastography for prostate cancer detection: Comparison with multiparametric MRI. Radiology 299:362-370
8. Park SJ, Yoon JH, Lee DH, Lim WH, Lee JM (2021) Tumor stiffness measurements on MR elastography for single nodular hepatocellular carcinomas can predict tumor recurrence after hepatic resection. J Magn Reson Imaging 53:587-596
9. Shahryari M, Tzschatzsch H, Guo J et al (2019) Tomoelastography Distinguishes Noninvasively between Benign and Malignant Liver Lesions. Cancer Res 79:5704-5710
10. Thompson SM, Wang J, Chandan VS et al (2017) MR elastography of hepatocellular carcinoma: Correlation of tumor stiffness with histopathology features-Preliminary findings. Magn Reson Imaging 37:41-45
11. Wang J, Shan Q, Liu Y et al (2019) 3D MR elastography of hepatocellular carcinomas as a potential biomarker for predicting tumor recurrence. J Magn Reson Imaging 49:719-73.
12. Milosevic MF, Pintilie M, Hedley DW et al (2014) High tumor interstitial fluid pressure identifies cervical cancer patients with improved survival from radiotherapy plus cisplatin versus radiotherapy alone. Int J Cancer 135:1692-1699
13. Bockelmann LC, Schumacher U (2019) Targeting tumor interstitial fluid pressure: Will it yield novel successful therapies for solid tumors? Expert Opin Ther Targets 23:1005-1014
Figures