2580
Can we evaluate the progression from acute kidney injury to chronic kidney disease with sodium MRI and disease biomarkers?1Department of Clinical Medicine, MR Research Centre, Aarhus University, Aarhus N, Denmark
Synopsis
This study evaluates 23Na-MRI and renal injury disease biomarkers in a porcine model of unilateral renal ischemia-reperfusion injury. Pigs were distributed into groups of healthy controls, 2 ischemia times, and three survival times (n = 25). We found a significant difference between thecorticomedullary sodium gradients in pigs exposed to long ischemia. Neutrophil gelatinase-associated lipocalin (NGAL) was increased immediately after the ischemia-reperfusion injury regardless of the length of ischemia, but only remained elevated in the long-time ischemia group. Our results suggest a kidney injury model with the ability to differentiate between reversible and progressive acute kidney injury.
Introduction
Acute kidney injury (AKI) is a frequent complication to a broad spectrum of diseases,[1-3] and AKI can cause development or progression of chronic kidney disease (CKD)[4, 5]. CKD affects approximately 10% of the world’s population[6]. Thus, the AKI-CKD transition is a topic with need for further investigation. Focus on sodium magnetic resonance imaging (23Na-MRI) has increased as a potential clinical MRI modality for evaluation of kidney tissue. 23Na-MRI has been used for noninvasive evaluation of the corticomedullary sodium gradient in rodent studies[7], as well as dynamic quantification of sodium biodistribution across the porcine and the human kidney.[8] Distribution of sodium in the kidney and the sodium gradient along the corticomedullary axis are a fundamental physiological high energy demanding process controlling the reabsorption of water and solutes. This function is a vital part of renal function and in turn renal dysfunction[7, 9]. Thus, 23Na-MRI has the potential to serve as a clinical tool for accurate diagnostic and treatment evaluation of the transition from AKI to CKD. In this study, we aimed to evaluate 23Na-MRI as biomarker for the AKI-CKD transition.Method
25 pigs were included in this study on kidney ischemia-reperfusion injury (IRI). IRI was induced to the right kidney for either 45 min (five pigs, mild AKI) or 120 min (15 pigs, severe AKI)[10]. Five pigs served as healthy. Ischemia was performed using a catheter-based approach through the femoral artery. A balloon was inflated in the right renal artery to induce ischemia, and reperfusion was obtained by deflation the balloon. All steps were performed with the use of fluoroscopy. Proton and sodium MRI was performed on day 1, 4 or 7 after the IRI on a 3T system (GE MR750, GE Healthcare) equipped with a sodium coil (Helmholtz loop, PulseTeq Limited, UK). Sodium images were acquired using a radial 3D sequence (field of view = 350 mm3, matrix size = 35, flip angle = 20°, TR/TE = 5.3/0.3 ms, 32 averages). Blood was taken before the IRI (baseline), 15 min after, and on the MRI scan day and analyzed for neutrophil gelatinase-associated lipocalin (NGAL). Tissue samples were taken for histology analysis. MR images were analyzed using Horos (The Horos Project, Horosproject.org) using semi-circular regions of interest drawn on proton images. The sodium level in cortex and medulla was normalized to the level of sodium in the blood in aorta, and the corticomedullary sodium gradient was normalized to the outer cortex of the same kidney. All statistical analyses were performed in GraphPad Prism 9.Results
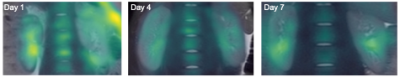
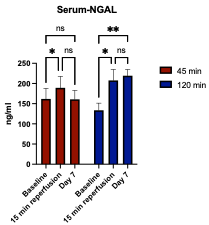
We were able to image the intra-renal sodium gradient from the cortex and medulla following either a 45 min or 120 min ischemia time in unilateral renal ischemia-reperfusion injury (Fig. 1). A statistically significant difference in the intra-renal sodium gradient was found 4 days after the injury (p = 0.0225) in the 120 min ischemia group (Fig. 2). A short ischemia time of 45 min did not reach statistical significance (p = 0.1020), (Fig. 2). These findings were supported by the biochemical kidney injury assay on serum NGAL, which increased 15 min after reperfusion in both groups, while it increased further on day 7 in the 120 min group and normalized in the 45 min group (Fig. 3).Discussion
We were able to perform sodium MRI in a pig model of AKI and support these findings using the kidney injury biomarker NGAL. Assuming an equal sodium gradient between healthy kidneys, difference between the sodium gradient in kidneys of the same individual might reflect alterations or deviations of the corticomedullary sodium axis. An altered sodium axis in the pigs exposed to renal injury probably reflects a compensatory mechanism of the healthy kidney or an impaired function of the ischemic kidney. One or both of these altered mechanisms might have occurred in the pigs exposed to long ischemia time on day 4 due to the difference in the corticomedullary sodium axis between the ischemic and the healthy kidney. Although it did not reach statistical significance, this compensatory mechanism was possibly maintained on day 7 in the group of 120 min ischemia, where the sodium gradient of the healthy kidney was magnified.Production of NGAL is triggered by tissue injury and clinical studies have shown that expression of NGAL in serum and urine is linked to impairment of kidney function.[11] The level of NGAL increases in both AKI and CKD patients, though it is not able to distinguish the conditions yet.[12] Our results show increased levels of serum-NGAL after an ischemia-reperfusion injury regardless of the length of ischemia time. The long ischemia time (120 min) might induce persisting or progressive kidney injury as the level of NGAL increased further on day 7, whereas the short ischemia time (45 min) resulted in a decreased level of NGAL on day 7, suggesting a reversible or mild stage of AKI.
Conclusion
23Na-MRI shows potential for evaluation of intrarenal sodium levels and more specific alterations in the corticomedullary sodium axis after an ischemia-reperfusion injury in pigs. Serum-NGAL enabled differentiation between the severity of kidney injury. Our results facilitate a promising foundation for further studies of the transition from AKI to CKD.Acknowledgements
This work was supported by the Lundbeck Foundation.References
1 Lankadeva, Y. R., Kosaka, J., Evans, R. G., Bellomo, R. & May, C. N. Urinary Oxygenation as a Surrogate Measure of Medullary Oxygenation During Angiotensin II Therapy in Septic Acute Kidney Injury. Crit Care Med 46, e41-e48, doi:10.1097/ccm.0000000000002797 (2018).
2 Ma, S. et al. Sepsis-induced acute kidney injury: A disease of the microcirculation. Microcirculation 26, e12483, doi:10.1111/micc.12483 (2019).
3 Walsh, M. et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology 119, 507-515, doi:10.1097/ALN.0b013e3182a10e26 (2013).
4 Coca, S. G., Yusuf, B., Shlipak, M. G., Garg, A. X. & Parikh, C. R. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis53, 961-973, doi:10.1053/j.ajkd.2008.11.034 (2009).
5 Hsu, R. K. & Hsu, C. Y. The Role of Acute Kidney Injury in Chronic Kidney Disease. Semin Nephrol 36, 283-292, doi:10.1016/j.semnephrol.2016.05.005 (2016).
6 Humphreys, B. D. Mechanisms of Renal Fibrosis. Annu Rev Physiol 80, 309-326, doi:10.1146/annurev-physiol-022516-034227 (2018).
7 Maril, N., Margalit, R., Mispelter, J. & Degani, H. Sodium magnetic resonance imaging of diuresis: spatial and kinetic response. Magn Reson Med 53, 545-552, doi:10.1002/mrm.20359 (2005).
8 Grist, J. T. et al. Visualization of sodium dynamics in the kidney by magnetic resonance imaging in a multi-site study. Kidney Int 98, 1174-1178, doi:10.1016/j.kint.2020.04.056 (2020).
9 Palmer, L. G. & Schnermann, J. Integrated control of Na transport along the nephron. Clin J Am Soc Nephrol 10, 676-687, doi:10.2215/cjn.12391213 (2015).
10 Orvieto, M. A. et al. Defining maximal renal tolerance to warm ischemia in porcine laparoscopic and open surgery model. Urology 66, 1111-1115, doi:10.1016/j.urology.2005.05.027 (2005).
11 Ntrinias, T. et al. Biomarkers in Progressive Chronic Kidney Disease. Still a Long Way to Go. Pril (Makedon Akad Nauk Umet Odd Med Nauki) 40, 27-39, doi:10.2478/prilozi-2020-0002 (2019).
12 Corbacıoglu, S. K. et al. Value of plasma neutrophil gelatinase-associated lipocalin (NGAL) in distinguishing between acute kidney injury (AKI) and chronic kidney disease (CKD). Turk J Emerg Med17, 85-88, doi:10.1016/j.tjem.2017.03.002 (2017).
Figures