2578
MRI-based Assessment of Urethral Biomechanics During Voiding1Radiology, University of Wisconsin-Madison, MADISON, WI, United States, 2Mechanical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 3Urology, University of Wisconsin-Madison, MADISON, WI, United States, 4Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
Non-invasive methods to evaluate voiding dysfunction in men are extremely limited. We report here a non-invasive, MRI-based protocol to characterize urethral biomechanics during voiding. All four subjects were able to void in the scanner with high-fidelity, 3D images of the urethra. These images were successfully analyzed for anatomy, function, and biomechanics. Future work will be aimed at furthering these methods and resulting metrics so that they may be applied clinically.
Introduction
The urethral anatomy and biomechanics during voiding have not been well studied. This is of considerable importance to achieve a better understanding of voiding dysfunction in aging men and the effect of urethral stricture disease on voiding dynamics. The commonly used diagnostic procedures of cystoscopy, retrograde urethrography and multichannel urodynamics with fluoroscopic imaging (video-urodynamics) are invasive and limited in the information they provide1,2. Building on our previously published work demonstrating the capabilities of magnetic resonance imaging (MRI) to provide 3D anatomical information of the bladder3,4, we examined the potential for a non-invasive, comprehensive MRI protocol to characterize urethral anatomy, function, and biomechanics during voiding.Methods
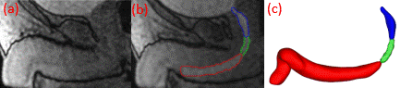
In this HIPAA compliant, IRB-approved study, 4 healthy males ages 29, 55, 62, 74 (subjects 1-4 respectively) were recruited to void in an MRI scanner. All scans were completed on a clinical 3T scanner (Premier, GE Healthcare, Waukesha, WI), using a high-density flexible surface coil array (AIR Coil, GE Healthcare). The dynamic acquisition was collected using 3D Differential Subsampling with Cartesian Ordering (DISCO) Flex and a temporal resolution of 3.7. 15 minutes prior to the MRI scans, 1/3 of a single weight-based dose (0.1 mmol/kg) of gadolinium-based contrast was slowly hand injected intravenously into each subject. Right before the scan, the subject applied a condom catheter. The patient voided in the scanner, and dynamic imaging of the urethra was collected throughout the void. After urination, a fast-spin echo T2-weighted axial acquisition with an in-plane resolution of 0.625mm and a slice thickness of 4mm. was performed in order to get anatomical reference images of the prostate. These images were imported into Mimics (Materialise, Leuven, Belgium), and using Mimics, urethra length and diameter at the internal and external urethral sphincters were measured. The urethra (divided into prostatic, membranous, and penile) was segmented (Figure 1b) at each time point of the dynamic imaging, while the prostate was segmented from the anatomical reference images. 3D renderings of the urethra were calculated (Figure 1c) to create a centerline. Using the centerline with the 3D renderings allowed for the acquisition of ellipticity (the degree of deviation from circularity) and best-fitted diameter through the entirety of the urethra. The average best-fitted diameter was calculated at each time step and for all three urethral sections. While the average ellipticity of the entire urethra was only acquired when the flow was at its maximum. When the external sphincter closed, signaling an end of voiding, residual urine volume in the prostatic urethra was calculated from the 3D rendering. 3D renderings of the prostate were also created from the T2-weighted images to obtain prostate volume.Results
All men were able to void in a supine position in the scanner, and scan sessions rendered high fidelity, full field of view images of the urethra, as seen by the time-resolved sagittal images in Figure 1a. The average ellipticity for the four subjects was between 0.47 and 0.64. The urethra length for subjects 1-4 are 23.5cm, 22.6cm, 22.2cm, and 23.2cm, respectively, while the average best-fitted diameter for each section of their respective urethras is displayed over time across the voiding cycle in Figure 2. The prostatic and penile sections had a greater diameter than the membranous section across the voiding cycle for all four subjects. The internal and external urethral sphincter diameters are shown in Figure 3, with the diameter of the internal sphincter generally being larger throughout the voiding cycle. It should also be noted that the internal sphincter did not always close at the same time as the external sphincter. This resulted in residual urine in the prostatic urethra that had volumes of 0cc, 0.89cc, 2.21cc, and 0.86cc, for subjects 1-4, respectively. The prostate volume for the four subjects 1-4 were 22.4cc, 36.2cc, 49.4cc, and 16.6cc, respectively.Discussion
Understanding of urethral function and biomechanics has been limited by the lack of suitable methods of study. This work demonstrates the feasibility of using MRI to acquire a detailed 3-D assessment of urethral anatomy, function, and biomechanics during voiding in a non-invasive fashion. In this ongoing study, more subjects will be recruited, and correlations between prostate size and urethral dimensions will be explored. These analysis methods and resulting metrics may be of great value when determining the role of urethral anatomy and biomechanics in normal voiding in men with voiding dysfunction or urethral stricture disease.Conclusion
MRI is an imaging modality that can be used to characterize anatomical and functional information of the urethra throughout the voiding cycle in a safe, accurate, and reproducible way. This study demonstrates the application of this protocol, while future studies will be aimed at applying and furthering these methods to a larger cohort of normal men and men with lower urinary tract dysfunction.Acknowledgements
The authors would like to acknowledge GE Healthcare and support from the NIH (R01 DK126850-01)References
[1] Verla, W., Oosterlinck, W., Spinoit, A. F., & Waterloos, M. (2019). A Comprehensive Review Emphasizing Anatomy, Etiology, Diagnosis, and Treatment of Male Urethral Stricture Disease. BioMed research international, 2019, 9046430. https://doi.org/10.1155/2019/9046430
[2] Engelsgjerd, J. S., & Deibert, C. M. (2021). Cystoscopy. In StatPearls. StatPearls Publishing
[3] Anzia, L. E., Johnson, C. J., Mao, L., Hernando, D., Bushman, W. A., Wells, S. A., & Roldán-Alzate, A. (2021). Comprehensive non-invasive analysis of lower urinary tract anatomy using MRI. Abdominal radiology (New York), 46(4), 1670–1676. https://doi.org/10.1007/s00261-020-02808-9
[4] Pewowaruk, R., Rutkowski, D., Hernando, D., Kumapayi, B. B., Bushman, W., & Roldán-Alzate, A. (2020). A pilot study of bladder voiding with real-time MRI and computational fluid dynamics. PloS one, 15(11), e0238404. https://doi.org/10.1371/journal.pone.0238404
Figures