2574
Frequency, pattern, and associations of renal iron accumulation in sickle/beta-thalassemia1Fondazione G. Monasterio CNR-Regione Toscana, Pisa, Italy, 2Azienda Ospedaliera di Rilievo Nazionale "A. Cardarelli", Napoli, Italy, 3Ospedale "Sandro Pertini", Roma, Italy, 4"ARNAS" Civico, Di Cristina Benfratelli, Palermo, Italy, 5Ospedale del Delta, Lagosanto (FE), Italy, 6Gemelli Molise SpA, Fondazione di Ricerca e Cura "Giovanni Paolo II", Campobasso, Italy, 7Presidio Ospedaliero "Giovanni Paolo II" - Distretto AG2 di Sciacca, Sciacca (AG), Italy, 8Presidio Ospedaliero Locri - A.S.P di Reggio Calabria, Locri (RC), Italy, 9Ospedale "Engles Profili", Fabriano, Italy
Synopsis
Sickle/beta-thalassemia and homozygous sickle cell disease (HbSS) patients were rather similar in respect to hepatic, pancreatic and cardiac iron deposition detected by the T2* MRI technique, but sickle/β-thalassemia patients had significantly less renal iron overload. In sickle/beta-thalassemia renal T2* values were not associated to age, gender, splenectomy, transfusions, serum ferritin levels or iron load in the liver, pancreas and heart, suggesting that performing MRI T2* in other organs is not a reliable approach to predict the exact renal iron state. Renal T2* values were inversely correlated with serum lactate dehydrogenase, confirming that kidney iron results from chronic hemolysis.
Introduction
Chronically transfused homozygous sickle cell disease (HbSS) patients were shown to have higher kidney iron deposition than thalassemia major patients, not associated to total body iron and mainly caused by chronic hemolysis1. Kidney iron deposition has not been explored in sickle/beta-thalassemia (Sβ-thal), resulting from the inheritance of both sickle cell and beta-thalassemia genes.This multicenter study aimed to study frequency, pattern, and associations of renal iron accumulation in sickle beta-thalassemia.
Methods
Thirty-three Sβ-thal patients (36.49±14.72 years; 13 females) consecutively enrolled in the Extension-Myocardial Iron Overload in Thalassemia (E-MIOT) network were considered. Moreover, 20 healthy subjects, 14 HbSS patients and 71 thalassemia major (TM) patients were included as comparison groups.Hepatic2, cardiac3, pancreatic4, and renal5 iron overload was quantified by the gradient-echo T2* technique. In each kidney T2* was measured in anterior, posterolateral, and posteromedial parenchymal regions and the global T2* value was calculated as the average of the two kidneys T2* values5.
Results
Global renal T2* were significantly higher in healthy subjects versus both Sβ-thal patients (49.68±10.09 ms vs 43.19±8.07 ms; P=0.013) and HbSS patients (49.68±10.09 ms vs 26.21±17.07 ms; P<0.0001).Sβ-thal patients showed comparable age, sex, frequency of regular transfusion, hematochemical parameters, and hepatic, cardiac and pancreatic iron load than HbSS patients, but they had a significant lower frequency of renal iron overload (global renal T2*<31 ms) (9.1% vs 57.1%; P=0.001).
Regularly transfused patients (16 Sβ-thal and 10 HbSS) were compared with TM patients, homogeneous for age and sex, but TM started regular transfusions significantly earlier and they were more frequently chelated. No significant difference was detected in terms of hepatic and cardiac iron levels, but TM patients had significantly lower pancreas T2* values than both the other two groups and significantly higher global renal T2* values than HbSS patients (42.87±9.43 ms vs 24.39±15.74 ms; P=0.001).
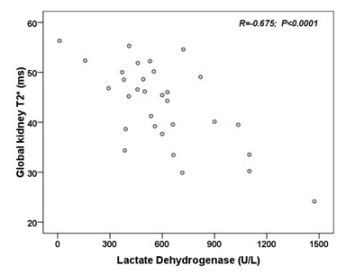
In Sβ-thal patients no significant difference was detected between T2* values in left and right kidneys, and global renal T2* values were not associated to age, gender, splenectomy, and they were comparable between regularly transfused and non transfused patients. No correlation was detected between renal T2* values and serum ferritin levels or iron load in the other organs. Global renal T2* values were not associated with serum creatinine levels but showed a significant inverse correlation with serum lactate dehydrogenase (Figure 1).
Conclusions
Renal iron deposition is not common in Sβ-thal patients, with a prevalence significantly lower compared to that of HbSS patients, but with a similar underlying mechanism due to the chronic hemolysis.Acknowledgements
We would like to thank all the colleagues involved in the E-MIOT project (https://emiot.ftgm.it/). We thank Silvia Miconi for her skillful secretarial work and all healthy subjects and patients for their cooperation.References
1. Schein A, Enriquez C, Coates TD, Wood JC. Magnetic resonance detection of kidney iron deposition in sickle cell disease: a marker of chronic hemolysis. J Magn Reson Imaging. 2008;28(3):698-704.
2. Positano V, Salani B, Pepe A, et al. Improved T2* assessment in liver iron overload by magnetic resonance imaging. Magn Reson Imaging. 2009;27(2):188-197.
3. Pepe A, Positano V, Santarelli F, et al. Multislice multiecho T2* cardiovascular magnetic resonance for detection of the heterogeneous distribution of myocardial iron overload. J Magn Reson Imaging. 2006;23(5):662-668.
4. Meloni A, De Marchi D, Positano V, et al. Accurate estimate of pancreatic T2* values: how to deal with fat infiltration. Abdom Imaging. 2015;40(8):3129-3136.
5. Grassedonio E, Meloni A, Positano V, et al. Quantitative T2* magnetic resonance imaging for renal iron overload assessment: normal values by age and sex. Abdominal Imaging. 2015;40:1700-1704.