2564
Graph theory demonstrates functional reorganization dynamics related to tumor grade and location in glioma
Luca Pasquini1,2, Mehrnaz Jenabi3, Kyung Peck4, and Andrei Holodny1
1Radiology, Memorial Sloan Kettering Cancer Center, New York City, NY, United States, 2NESMOS, La Sapienza University, Rome, Italy, 3Radiology, Memorial Sloan Kettering Cancer Center, New York CIty, NY, United States, 4Medical Physics, Memorial Sloan Kettering Cancer Center, New York City, NY, United States
1Radiology, Memorial Sloan Kettering Cancer Center, New York City, NY, United States, 2NESMOS, La Sapienza University, Rome, Italy, 3Radiology, Memorial Sloan Kettering Cancer Center, New York CIty, NY, United States, 4Medical Physics, Memorial Sloan Kettering Cancer Center, New York City, NY, United States
Synopsis
Brain tumors lead to modifications of brain networks known as functional reorganization or plasticity, which may be compensatory in nature and lead to valuable clinical applications. However, the determinants of plasticity are unclear. We evaluated functional networks of 30 low-grade (LGG) and 30 high-grade (HGG) left-hemispheric gliomas versus 20 healthy controls (HC) through rs-fMRI and graph-theory. We hypothesized that tumor grade and location impact brain connectivity. Our results show that both LGG and HGG develop ipsilateral and contralateral functional network changes. Additionally, tumor location is crucial: frontal and temporal tumors show bilateral modifications; parietal and insular tumors only local effects.
BACKGROUND
Functional reorganization represents an adaptive phenomenon of the brain to compensate tumor invasion(1). Network modifications have been demonstrated using resting-state functional MRI (rs-fMRI) in patients with brain tumors, possibly representing a correlate of plastic changes(1). These modifications may help compensating neurological deficits and/or enhance patient recovery after surgery(2). In the case of patients harboring brain tumors, a better understanding of functional reorganization may allow the enhancement of plastic phenomena to support surgical radicality(3). On the other hand, long-range functional modifications may be detrimental in other conditions such as diaschisis in stroke(4). Understanding the precise dynamics of functional reorganization in brain tumors, including the impact of tumor grade and location, is a prerequisite for clinical implementation. Our objective was to evaluate functional network changes induced by left-hemispheric low-grade (LGG) and high-grade (HGG) gliomas versus healthy controls (HC) by applying graph-theory on rs-fMRI. We hypothesized that tumor grade and location in the brain will have an effect on intra-hemispheric and inter-hemispheric functional connectivity.METHODS
We recruited patients following inclusion criteria: left-hemispheric glioma, no motion or susceptibility artifact, pre-operative rs-fMRI and 3D T1-weighted anatomical images. Matched HC were included for comparison. Four location labels were assigned to the tumors (frontal, temporal, parietal, insular). We performed functional connectivity analyses with CONN(5), as follows: functional and anatomical images were co-registered to the MNI space and parcellated (136 parcels); ROI-to-ROI connectivity matrices were obtained for whole brain, left and right hemisphere of HGG, LGG and HC groups. Seven graph-theoretical metrics were calculated for the resulting functional networks applying two-sided FDR correction (p<0.05): global/local efficiency, betweenness centrality, cost, average path length, clustering coefficient, degree. Two-tailed Student t-test was employed to compare graph-theoretical metrics in the study groups for: whole brain, left and right hemispheric networks. To account for tumor location effects, the same analysis was repeated for lobar nodes in subgroup of patients divided by location labels.RESULTS
Sixty patients (30 LGG, mean age 40Y, 21M; 30 HGG, mean age 61Y, 22M) and 20 HC (mean age 48Y) were included. Tumor location labeling demonstrated involvement of: frontal lobe 20/60 (5 HGG, 15 LGG), temporal lobe 29/60 (16 HGG, 13 LGG), parietal lobe 15/60 (11 HGG, 4 LGG), insula 15/60 (3 HG, 12 LGG). LGG showed significant differences in left vs right hemisphere functional network (global efficiency p=0.02; local efficiency p=0.01; clustering coefficient p=0.01). Also, the left hemispheric network was different in LGG compared to HC (global efficiency, p=0.03). HGG showed significant differences in the right hemispheric network compared to HC (cost, p=0.036). No significant differences emerged from comparing whole brain networks. Detailed results for tumor location-related comparisons are available in Table1-3. In brief, frontal tumors showed multiple network changes in the left and right hemisphere (p<0.05). Right-sided changes were located in the Superior Frontal Gyrus (SFG); left-sided changes were localized in the SFG, Middle Frontal Gyrus, Inferior Frontal Gyrus Triangular part. Temporal tumors showed multiple network changes in the left and right hemisphere (p<0.05). Right-sided changes were located in the Superior Temporal Gyrus (STG) posterior division, Middle Temporal Gyrus (MTG) posterior division, MTG temporo-occipital part, Inferior Temporal Gyrus (ITG) anterior division. Left-sided changes were located in STG anterior division, STG posterior division, MTG anterior division, ITG anterior division. Parietal and insular tumors showed network changes for both LGG and HGG in the left hemisphere only (p<0.05). Particularly, parietal tumors showed network changes in the Post-Central Gyrus and Angular Gyrus.DISCUSSION & CONCLUSION
Right-hemispheric functional changes are more expected in LGG than HGG(6). We showed that both LGG and HGG can develop ipsilateral and contralateral functional network changes, as captured by variation of graph-theoretical measures. The different impact of low vs high grade tumors on functional networks has often been related to the slower growth of LGG, which is supposed to allow long-range plastic changes. However, previous studies reported functional changes in HGG, for example in the cerebro-cerebellar language-related circuits(7) or language-related areas(8). In fact, the median age of glioblastomas at diagnosis is approximately 330 days(9), while brain modifications related to learning new functions may develop within months(10,11). This time-window may be enough to develop significant network modifications. The effect of tumor location on brain plasticity has rarely been investigated. From our results, tumor location seems crucial for network changes: while frontal and temporal tumors showed bilateral functional modifications, parietal and insular tumors displayed only local effects. Notably, primary language areas are lateralized to the left frontal and temporal lobes, which may suggest that inter-hemispheric reorganization favors primary vs secondary eloquent areas. In fact, damage to eloquent regions (connectors) leads to deeper network modifications than peripheral areas(12), in agreement with neuro-computational models(13).The compensatory nature and clinical meaning of functional modifications induced by brain tumors of different grade and location remains unclear. Future studies may elucidate this aspect, with possible effects on targeted therapies, as well as patient’s quality and quantity of life.Acknowledgements
We thank RSNA foundation for supporting this project through the grant RF2109References
- Cargnelutti E, Ius T, Skrap M, Tomasino B. NeuroImage : Clinical What do we know about pre- and postoperative plasticity in patients with glioma ? A review of neuroimaging and intraoperative mapping studies. NeuroImage: Clinical (2020) 28:102435. doi:10.1016/j.nicl.2020.1024352.
- Picart T, Herbet G, Moritz-Gasser S, Duffau H. Iterative Surgical Resections of Diffuse Glioma with Awake Mapping: How to Deal with Cortical Plasticity and Connectomal Constraints? Clinical Neurosurgery (2019) 85:105–116. doi:10.1093/neuros/nyy2183.
- Rivera-Rivera PA, Rios-Lago M, Sanchez-Casarrubios S, Salazar O, Yus M, González-Hidalgo M, Sanz A, Avecillas-Chasin J, Alvarez-Linera J, Pascual-Leone A, et al. Cortical plasticity catalyzed by prehabilitation enables extensive resection of brain tumors in eloquent areas. Journal of Neurosurgery (2017) 126:1323–1333. doi:10.3171/2016.2.JNS1524854.
- Corbetta M, Ramsey L, Callejas A, Baldassarre A, Hacker CD, Siegel JS, Astafiev S v., Rengachary J, Zinn K, Lang CE, et al. Common behavioral clusters and subcortical anatomy in stroke. Neuron (2015) 85:927–941. doi:10.1016/j.neuron.2015.02.0275.
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connectivity (2012) 2:125–141. doi:10.1089/brain.2012.00736.
- Desmurget M, Bonnetblanc F, Duffau H. Contrasting acute and slow-growing lesions: A new door to brain plasticity. Brain(2007) 130:898–914. doi:10.1093/brain/awl3007.
- Zhang N, Xia M, Qiu T, Wang X, Lin C po, Guo Q, Lu J, Wu Q, Zhuang D, Yu Z, et al. Reorganization of cerebro-cerebellar circuit in patients with left hemispheric gliomas involving language network: A combined structural and resting-state functional MRI study. Human Brain Mapping (2018) 39:4802–4819. doi:10.1002/hbm.243248.
- Briganti C, Sestieri C, Mattei PA, Esposito R, Galzio RJ, Tartaro A, Romani GL, Caulo M. Reorganization of functional connectivity of the language network in patients with brain gliomas. American Journal of Neuroradiology (2012) 33:1983–1990. doi:10.3174/ajnr.A30649.
- Stensjøen AL, Berntsen EM, Jakola AS, Solheim O. When did the glioblastoma start growing, and how much time can be gained from surgical resection? A model based on the pattern of glioblastoma growth in vivo. Clinical Neurology and Neurosurgery (2018) 170:38–42. doi:10.1016/j.clineuro.2018.04.02810.
- Mårtensson J, Eriksson J, Bodammer NC, Lindgren M, Johansson M, Nyberg L, Lövdén M. Growth of language-related brain areas after foreign language learning. NeuroImage (2012) 63:240–244. doi:10.1016/j.neuroimage.2012.06.04311.
- Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, Büchel C, May A. Temporal and spatial dynamics of brain structure changes during extensive learning. The Journal of neuroscience : the official journal of the Society for Neuroscience (2006) 26:6314–6317. doi:10.1523/JNEUROSCI.4628-05.200612.
- Gratton C, Nomura EM, Pérez F, D’Esposito M. Focal brain lesions to critical locations cause widespread disruption of the modular organization of the brain. Journal of Cognitive Neuroscience (2012) 24:1275–1285. doi:10.1162/jocn_a_0022213.
- Keidel JL, Welbourne SR, Lambon Ralph MA. Solving the paradox of the equipotential and modular brain: A neurocomputational model of stroke vs. slow-growing glioma. Neuropsychologia (2010) 48:1716–1724. doi:10.1016/j.neuropsychologia.2010.02.019
Figures
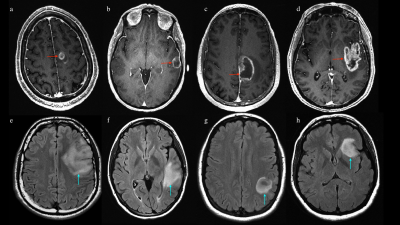
Images above: post-contrast 3d T1-weighted MR scans of 4 patients with high-grade glioma (image a-d, red arrows). Tumor location was labeled to account for involved lobes as follows: frontal (a), temporal (b), parietal (c) and insular involvement (d). Images below: FLAIR-weighted MR scans of 4 patients with low-grade glioma (image e-h, light blue arrows). Tumor location was labeled to account for involved lobes as follows: frontal (e), temporal (f), parietal (g) and insular involvement (h).
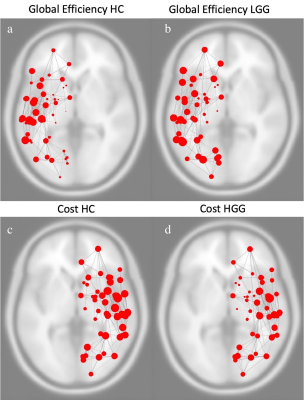
Images above represent global efficiency of left hemispheric functional network in healthy controls (a) and patients with log-grade glioma (b). Images below represent cost of right hemispheric functional network in healthy controls (c) and patients with high-grade glioma (d).
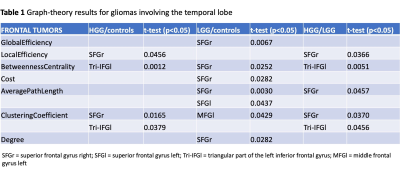
Summary of graph-theory results for gliomas involving the frontal lobe
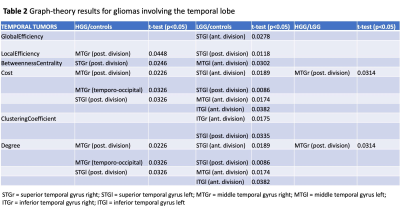
Summary of graph-theory results for gliomas involving the temporal lobe
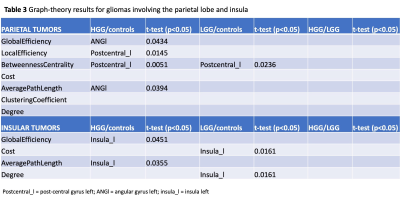
Summary of graph-theory results for gliomas involving the parietal lobe and insula
DOI: https://doi.org/10.58530/2022/2564