2563
The vestibular neuromatrix in patients with post-concussive vestibular dysfunction and healthy controls1Radiology and Imaging Sciences, Emory University School of Medicine, Atlanta, GA, United States, 2Georgia State University, Atlanta, GA, United States, 3Center for Advanced Brain Imaging, Georgia Institute of Technology, Atlanta, GA, United States, 4Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory University, Atlanta, GA, United States, 5Shepherd Center, Atlanta, GA, United States, 6Neurology, Emory University School of Medicine, Atlanta, GA, United States
Synopsis
Convergent clinical and neuroimaging evidence suggests that cognitive-affective and vestibular symptoms are interrelated: affective disorders not only co-occur with vestibular dysfunction but may also influence vestibular processing. However, the topology of the vestibular/cognitive/affective network (‘vestibular neuromatrix’) is not well-defined. The present study leveraged graph theory metrics to assess the functional and structural connectivity among 82 regions of interest in healthy controls and in patients with subacute post-concussive vestibular dysfunction. Patients exhibited deficiencies in connectivity among vestibular, pre- and orbitofrontal, and visual regions, as well as in the integration of visual and vestibular information, visuospatial attention, and monitoring of internal state.
Introduction
The central vestibular network1-7 does not function in isolation but is likely interconnected with regions subserving cognitive and affective state.6 Anxiety, depression, and other affective disorders not only co-occur with vestibular dysfunction, but may also modulate vestibular systems themselves,8-12 and vestibular functions may influence conceptualization of body representation and ownership in addition to aiding spatial cognition and interoception.13,14 Clinical vestibular syndromes may therefore result in deficits across a variety of functional domains.15,16 Here, we leverage structural and functional neuroimaging to characterize this neuromatrix in healthy control participants and patients with post-concussive vestibular dysfunction (PCVD).Methods
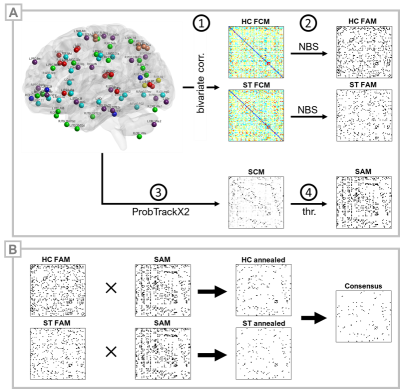
This IRB-approved cohort study included 23 patients with subacute PCVD (ST) and 35 healthy control subjects (HC). ST inclusion criteria were: age >16 years; no history of moderate or severe head injury, intracranial hemorrhage, seizure disorder, musculoskeletal injury affecting gait or balance, prior neurological surgery, chronic drug or alcohol use, or abnormal head impulse testing or video nystagmography consistent with peripheral vestibular hypofunction or benign paroxysmal positional vertigo; a diagnosis of concussion17,18 occurring 4-12 weeks prior to enrollment; and clinical evidence for vestibular impairment as indicated by abnormal VOMS scores.16 Brain MRI acquisitions included a high-resolution 3D T1-MPRAGE, multiband resting-state fMRI (TR 750ms, TA 7.5min), and diffusion-weighted imaging (4 shells, 128 directions) on a 3T Siemens Prismafit MRI. 82 spherical (ø=8mm) MNI-space ROIs, comprising putative cortical substrates of spatial localization, orientation, saccadic, affective,19,20 and vestibular functions,1,21-31 including parieto-insular vestibular cortex (PIVC/OP21), multisensory orientation areas (MSO1), and the visual-vestibulomotor convergence zone (VVMCZ29), were selected for analysis. rsfMRI preprocessing was performed using minimal processing pipelines in the CONN Toolbox v19c.32 Bivariate correlations were Fisher-transformed to Z-scores and stored in subject-specific 82×82 functional connectivity matrices (FCMs). Functional adjacency matrices (FAMs) were generated by one-sample, within-group tests on FCMs and corrected for network FWER and connection FDR using network-based statistics.33 Additionally, groupwise FAMs were constrained by a structural connectivity matrix (SCM) to yield ‘HC-annealed’ and ‘ST-annealed’ matrices. Diffusion images were first processed using standard pipelines and tools (TOPUP, EDDY, BedpostX) within FSL34 and an 82×82 SCM computed per subject using ProbTrackX235 (5000 streamlines per seed voxel). The group mean SCM was thresholded using a replication method36-38 to produce a binarized structural adjacency matrix (SAM). Connections present in both HC and ST annealed matrices were considered part of a ‘consensus’ network (Figure 1). Normalized betweenness centrality was assessed for binary-directed graphs using the Brain Connectivity Toolbox.39Results
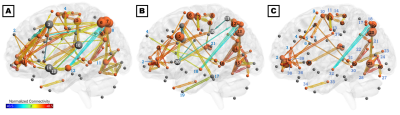
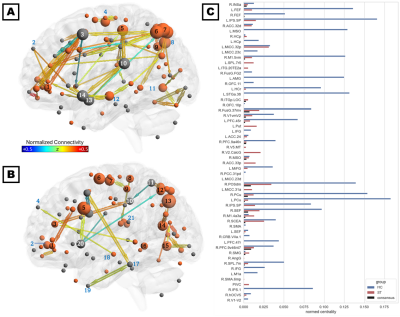
The consensus network (Figure 2C) was predominantly localized to the right hemisphere and included connections within and between four subnetworks: (1) parieto-occipital connections, including early visual areas V1 and V2 and the dorsal and ventral visual stream; (2) motor/supplementary motor cortex, frontal/supplementary/cingulate eye fields, and the VVMCZ; (3) vestibular cortex and right anterior insula and supramarginal gyrus; and (4) pre/orbitofrontal cortex and anterior and middle cingulate. Mean correlations across all ROIs and participants were positive, except between left rostral hippocampus and the precuneus, which were anticorrelated. The HC-annealed network (Figure 2A) was bilaterally distributed, with anterior insula, frontal eye fields, pre/inferofrontal cortex, amygdala, anterior and middle cingulate areas, and left superior temporal area 38 (L STG38a) serving as bridging nodes between subnetworks. The largest HC betweenness centralities were observed in the inferior parietal sulcus (IPS) and precuneus, left STG38a, amygdala, frontal eye field, and MSO, and right M1. Conversely, left inferior frontal gyrus, amygdala, and STG38a did not serve as bridging nodes in ST participants, and connections among the right prefrontal, anterior insular, and anterior and middle cingulate cortices and the frontal eye fields were diminished relative to control participants. Additionally, ST participants exhibited several connections which were not present in HC, including putamen-anterior cingulate, putamen-IPS, SEF-M1, and SPL-middle cingulate (Figures 2B, 3A, and 3B). HC exhibited greater betweenness centrality than ST across all connections except the left putamen, SPL, and inferotemporal TE2a, and right posterior hippocampus, visual areas, angular and supramarginal gyri, PIVC, motor cortex, and anterior and middle cingulate (Figure 3C).Discussion
The present results indicate extant functional and structural connectivity between vestibular and visuospatial, attentional, motor, and affective networks in both healthy participants and those with subacute PCVD. Moreover, subacute (ST) participants exhibited a dis-integration of functional, but not structural, connectivity among frontal/prefrontal, parieto-occipital, vestibular, and cingulate subnetworks and diminished primacy of integrative areas including pre/orbitofrontal cortex and MSO. Compellingly, groupwise differences in connectivity and centrality of regions subserving spatial localization,27,28 visual, visuospatial attention, visuomotor coordination,40-44 default-mode,42,44-46 and central executive functions42,43,47 suggest that symptoms in some vestibular syndromes may emerge from dysfunction in the integration of visual and vestibular information, visuospatial attention, and monitoring of internal state. Additional analyses, both planned and in progress, will assess the predictive power of these network connectivities against clinical scores of vestibular function.Conclusion
Current findings suggest that subacute PCVD subjects exhibit dis-integration of subnetworks subserving attention and executive functions, internal state monitoring, vestibular and vestibular processing, and multimodal integration, as well as increased primacy of the anterior and middle cingulate, early visual cortex, and supplementary eye fields. Cognitive-affective regions, including the prefrontal and orbitofrontal cortex, anterior and subgenual cingulate, and insula, were functionally and structurally connected within both groups.Acknowledgements
This work was supported by the Radiological Society of North America (RSNA) [Resident Research Grant RR1866] and by seed grants from the Georgia State/Georgia Tech Center for Advanced Brain Imaging (CABI) Neural Engineering Center and the Emory University Department of Radiology and Imaging Sciences.References
1. zu Eulenburg, P., et al., Meta-analytical definition and functional connectivity of the human vestibular cortex. Neuroimage, 2012. 60(1): p. 162-9.
2. Dieterich, M. and T. Brandt, The bilateral central vestibular system: its pathways, functions, and disorders. Ann N Y Acad Sci, 2015. 1343: p. 10-26.
3. Kirsch, V., et al., Handedness-dependent functional organizational patterns within the bilateral vestibular cortical network revealed by fMRI connectivity based parcellation. Neuroimage, 2018. 178: p. 224-237.
4. Alsalman, O., et al., The Neural Correlates of Chronic Symptoms of Vertigo Proneness in Humans. PLoS One, 2016. 11(4): p. e0152309.
5. Indovina, I., et al., Structural connectome and connectivity lateralization of the multimodal vestibular cortical network. Neuroimage, 2020. 222: p. 117247.
6. Brandt, T., M. Strupp, and M. Dieterich, Towards a concept of disorders of “higher vestibular function”. Frontiers in integrative neuroscience, 2014. 8: p. 47.
7. Raiser, T.M., et al., The human corticocortical vestibular network. Neuroimage, 2020. 223: p. 117362.
8. Staab, J.P., The influence of anxiety on ocular motor control and gaze. Current opinion in neurology, 2014. 27(1): p. 118-124.
9. Passamonti, L., et al., Brain responses to virtual reality visual motion stimulation are affected by neurotic personality traits in patients with persistent postural-perceptual dizziness. Journal of Vestibular Research, 2018. 28(5-6): p. 369-378.
10. Boccia, M., et al., Different neural modifications underpin PTSD after different traumatic events: an fMRI meta-analytic study. Brain imaging and behavior, 2016. 10(1): p. 226-237.
11. Preuss, N., G. Hasler, and F.W. Mast, Caloric vestibular stimulation modulates affective control and mood. Brain stimulation, 2014. 7(1): p. 133-140.
12. Ponzo, S., et al., Balancing body ownership: Visual capture of proprioception and affectivity during vestibular stimulation. Neuropsychologia, 2018. 117: p. 311-321.
13. Lopez, C., The vestibular system: balancing more than just the body. Current opinion in neurology, 2016. 29(1): p. 74-83.
14. Mast, F.W., et al., Spatial cognition, body representation and affective processes: the role of vestibular information beyond ocular reflexes and control of posture. Frontiers in integrative neuroscience, 2014. 8: p. 44.
15. Allen, J.W., et al., Altered Processing of Complex Visual Stimuli in Patients with Postconcussive Visual Motion Sensitivity. AJNR Am J Neuroradiol, 2021.
16. Trofimova, A., et al., Alterations in Resting-State Functional Brain Connectivity and Correlations with Vestibular/Ocular-Motor Screening Measures in Postconcussion Vestibular Dysfunction. J Neuroimaging, 2021.
17. Kristman, V.L., et al., Methodological issues and research recommendations for prognosis after mild traumatic brain injury: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch Phys Med Rehabil, 2014. 95(3 Suppl): p. S265-77.
18. Carroll, L.J., et al., Methodological issues and research recommendations for mild traumatic brain injury: the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med, 2004(43 Suppl): p. 113-25.
19. Gudayol-Ferré, E., et al., Changes in brain connectivity related to the treatment of depression measured through fMRI: a systematic review. Frontiers in human neuroscience, 2015. 9: p. 582.
20. Mochcovitch, M.D., et al., A systematic review of fMRI studies in generalized anxiety disorder: evaluating its neural and cognitive basis. Journal of affective disorders, 2014. 167: p. 336-342.
21. Jamadar, S.D., J. Fielding, and G.F. Egan, Quantitative meta-analysis of fMRI and PET studies reveals consistent activation in fronto-striatal-parietal regions and cerebellum during antisaccades and prosaccades. Front Psychol, 2013. 4: p. 749.
22. Vernet, M., et al., Frontal eye field, where art thou? Anatomy, function, and non-invasive manipulation of frontal regions involved in eye movements and associated cognitive operations. Front Integr Neurosci, 2014. 8: p. 66.
23. DeSouza, J.F., R.S. Menon, and S. Everling, Preparatory set associated with pro-saccades and anti-saccades in humans investigated with event-related FMRI. J Neurophysiol, 2003. 89(2): p. 1016-23.
24. Berman, R.A., et al., Cortical networks subserving pursuit and saccadic eye movements in humans: an FMRI study. Hum Brain Mapp, 1999. 8(4): p. 209-25.
25. Kellar, D., et al., Comparing fMRI activation during smooth pursuit eye movements among contact sport athletes, non-contact sport athletes, and non-athletes. Neuroimage Clin, 2018. 18: p. 413-424.
26. Eickhoff, S.B., et al., Identifying human parieto-insular vestibular cortex using fMRI and cytoarchitectonic mapping. Hum Brain Mapp, 2006. 27(7): p. 611-21.
27. Blum, S., et al., Functional connectivity of the posterior hippocampus is more dominant as we age. Cogn Neurosci, 2014. 5(3-4): p. 150-9.
28. Poppenk, J., et al., Past experience modulates the neural mechanisms of episodic memory formation. J Neurosci, 2010. 30(13): p. 4707-16.
29. Della-Justina, H.M., et al., Interaction of brain areas of visual and vestibular simultaneous activity with fMRI. Exp Brain Res, 2015. 233(1): p. 237-52.
30. Kolster, H., R. Peeters, and G.A. Orban, The retinotopic organization of the human middle temporal area MT/V5 and its cortical neighbors. J Neurosci, 2010. 30(29): p. 9801-20.
31. Dumoulin, S.O., et al., A new anatomical landmark for reliable identification of human area V5/MT: a quantitative analysis of sulcal patterning. Cereb Cortex, 2000. 10(5): p. 454-63.
32. Whitfield-Gabrieli, S. and A. Nieto-Castanon, Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect, 2012. 2(3): p. 125-41.
33. Zalesky, A., A. Fornito, and E.T. Bullmore, Network-based statistic: identifying differences in brain networks. Neuroimage, 2010. 53(4): p. 1197-1207.
34. Zhu, D., et al., Fusing DTI and fMRI data: a survey of methods and applications. NeuroImage, 2014. 102: p. 184-191.
35. Behrens, T.E., et al., Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage, 2007. 34(1): p. 144-55.
36. Van Den Heuvel, M.P. and O. Sporns, Rich-club organization of the human connectome. Journal of Neuroscience, 2011. 31(44): p. 15775-15786.
37. Rosen, B.Q. and E. Halgren, A whole-cortex probabilistic diffusion tractography connectome. bioRxiv, 2020.
38. Buchanan, C.R., et al., The effect of network thresholding and weighting on structural brain networks in the UK Biobank. NeuroImage, 2020. 211: p. 116443.
39. Rubinov, M. and O. Sporns, Complex network measures of brain connectivity: uses and interpretations. Neuroimage, 2010. 52(3): p. 1059-69. 40. Seghier, M.L., The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist, 2013. 19(1): p. 43-61.
41. Uddin, L.Q., et al., Dissociable connectivity within human angular gyrus and intraparietal sulcus: evidence from functional and structural connectivity. Cereb Cortex, 2010. 20(11): p. 2636-46.
42. Rudorf, S., et al., Intrinsic connectivity networks underlying individual differences in control-averse behavior. Hum Brain Mapp, 2018. 39(12): p. 4857-4869.
43. Shirer, W.R., et al., Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex, 2012. 22(1): p. 158-65.
44. Damoiseaux, J.S., et al., Reduced resting-state brain activity in the "default network" in normal aging. Cereb Cortex, 2008. 18(8): p. 1856-64.
45. Pascual, B., et al., Large-scale brain networks of the human left temporal pole: a functional connectivity MRI study. Cereb Cortex, 2015. 25(3): p. 680-702.
46. Xu, X., H. Yuan, and X. Lei, Activation and Connectivity within the Default Mode Network Contribute Independently to Future-Oriented Thought. Sci Rep, 2016. 6: p. 21001. 47. Cole, D.M., et al., Orbitofrontal connectivity with resting-state networks is associated with midbrain dopamine D3 receptor availability. Cereb Cortex, 2012. 22(12): p. 2784-93.
Figures