2554
Numerical Simulation of Tissue Damage Due to Magnetically Induced Force for Multi-Configuration Passive Medical Devices1MR:comp GmbH, Gelsenkirchen, Germany, 2MRI-STaR - Magnetic Resonance Institute for Safety, Technology and Research GmbH, Gelsenkirchen, Germany
Synopsis
Numerical simulation was performed to evaluate tissue damage due to magnetically induced (MI) force in 3T Magnetic Resonance (MR) environment for multi-configuration passive medical devices. Static magnetic and mechanical simulations were employed to determine the impact of MI force on the tissue surrounding the implant. The simulation results were used to select the worst-case for passive medical devices and for quantitative assessment of the tissue damage.
Introduction
A magnetically induced displacement of a medical implant can cause hazard to a patient inside an MR scanner. Furthermore, the displacement of the implant can even lead to a fatal outcome1 especially if it consists of sharp metallic surfaces or ferromagnetic materials. Currently, the standard test for MR compatibility of passive implants consists in performing a qualitative assessment based on the experimentally measured ratio of the MI force to the gravitational force2. In general, the worst-case for multi-configuration passive medical devices is selected based on a rational approach, namely by considering the largest mass and the highest permeability, whereas the effect of a sharp geometry may be omitted. The implant may be labelled as an MR-safe device when the MI force is lower than the gravitational force. However, the entire impact of the MI force on the biological tissues is not covered completely in the currently-available physical test. It has been implicitly recommended in the current version of the FDA-guidance3 that a criterion of acceptance should address the tissue damage caused by the MI force, torque, and vibration. The experimental measurement of tissue damage is highly complex to be realized; therefore, a numerical simulation is proposed as a preliminary evaluation. In this contribution, a framework for simulating the tissue damage by using a commercial numerical-software is presented.Methods
Both static and mechanic solvers of the simulation platform 3DS CST Studio Suite 2019 (Darmstadt, Germany) were employed to assess the tissue damage. First, a static magnetic field was generated by modelling the cylindrical magnet using the static solver as shown in Fig. 1. An implantable clip with a predefined relative magnetic permeability (µr), that was located at the entrance of the cylindrical magnet, was considered as an object under test in simulations. The force density calculated from the interaction of the induced magnetic field and the clip was imported as the field source of the mechanic solver. Secondly, the clip surrounded by tissue was generated in the mechanic solver as shown in Fig. 2, where a displacement boundary was defined at the bottom of the tissue. In this scenario, two clips with different tip geometries were compared as illustrated in Fig. 3. It can be seen that the clip 2 has a sharper tip than the clip 1, whereas the remaining parameters were kept the same.Results and discussion
Simulation results shown in Fig. 4 were generated with µr = 500 to depict a significant effect of the MI force. The simulation result shows that the clip with the sharper tips could generate a higher risk, which is indicated by the larger displacement. Based on this simulation, the variation of geometry should be considered in the worst-case analysis instead of just selecting the largest mass. Since the maximum stress on the tissue was calculated higher than the tensile stress (the limit of irreversible damage), the tissue would have been damaged. Based on this study, the tissue damage information can be assessed by calculating displacement and comparing the calculated maximum stress with the tensile stress. However, further mechanical properties of the tissue, which show the limit of hazard, pain and sensitivity of the organ should be thoroughly investigated. Furthermore, the safety labelling for MR compatibility should be evaluated by considering tissue damage caused by the MI force, torque and vibration. Finally, the validation of the simulation based on an experimental test is required.Conclusion
A framework for predicting the tissue damage is proposed as a procedure to assess the worst-case MI force for multiple-configuration passive medical devices, which is not covered by the conventional test. The effect of different geometry in the product matrix could be determined numerically and the tissue damage could be estimated.Acknowledgements
No acknowledgement found.References
- R P Klucznik, DA Carrier, R Pyka, RW Haid. Radiology, Vol 187, 855-856, 1993.
- American Society for Testing and Materials. Standard Test Method for Measurement of Magnetically Induced Displacement Force on Medical Devices in the Magnetic Resonance Environment, 2015.
- U.S. Food and Drag Administration. Testing and Labelling Medical Devices for Safety in the Magnetic Resonance (MR) Environment, May 2021.
- Kuthe CD, Uddanwadiker RV, Ramteke A. Experimental evaluation of fiber orientation based material properties of skeletal muscle in tension. Molecular & Celluler Biomechanic. 2014 Jun;11(2):113-28.
- Suzan C.D., Erol C, Yunus Z.A. A review of finite element applications in oral and maxillofacial biomechanics. Journal of Mechanics in Medicine and Biology Vol. 18, No. 2, 2018.
- http://www.matweb.com. Accessed November 10, 2021.
Figures
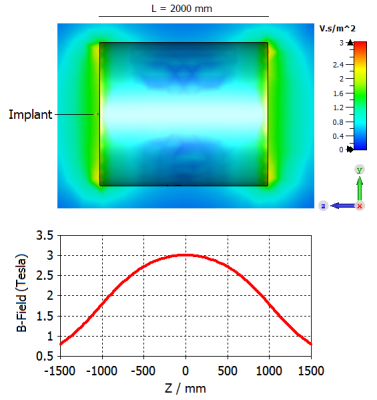
Fig. 1: Model of cylindrical magnet with sagittal plot at x = 0 (top); B-field background along z-axis at x = 0 and y = 0 (bottom)
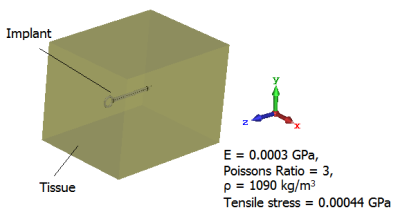
Fig. 2: Model of tissue surrounding implant, E = modulus elasticity, ρ = mass density
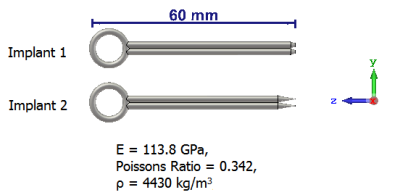
Fig. 3: Generic clips
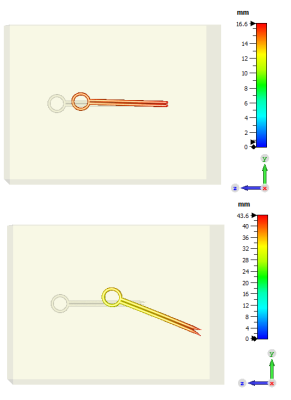
Fig. 4: Displacement in the tissue: implant 1 (top), maximum first principal stress = 2.79 GPa; implant 2 (bottom), maximum first principal stress = 8.16 GPa