2551
Evaluation of SAR and heating in children exposed to a 7T MRI Head Coil
Shaihan J Malik1,2, David J Carmichael1, Jeffrey W Hand1, and Joseph V Hajnal1,2
1Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom
1Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom
Synopsis
Electromagnetic and thermal simulations of a range of models (body mass 14-70 kg) were performed for a 7T (297MHz) birdcage head transmit coil. Head averaged SAR (hdSAR) was found to be the limiting factor for all cases, and a (weak) negative linear correlation with body mass was observed (i.e. hdSAR increases with decreasing body mass). Thermal simulations with fixed blood temperature (assumes good thermoregulation) predict temperature increases remain within guidelines if SAR limits are respected. If blood temperature is allowed to vary then elevated core temperatures are predicted for subjects with smaller body mass even when SAR limits are respected.
Introduction
Although human 7 T MRI scanners have been available for over a decade, clinical use is not mainstream, in part, because scanners have lacked regulatory approval. For example one model obtained European and US approval for clinical use for patients with mass > 30 kg1. With the aim of assessing the feasibility of 7 T scanning for subjects < 30 kg, this study evaluates specific absorption rate (SAR) and temperature distributions resulting from exposure to a 297MHz head transmit coil across a wide range of body mass.Methods
A generic single channel transmit shielded birdcage coil (16 rungs, length/diameter = 190mm/305mm for the coil and 205mm/370mm for the shield) was simulated using Sim4Life v5.2.2.1924 (Zurich MedTech AG, Zurich, Switzerland) with a total of 8 voxel models consisting of 7 children (ages 3-14) and one adult male (“Duke”) taken from the Virtual Population2: full details are available in Table 1. The coil was tuned and matched on the adult. Each model was positioned with brain centred in the coil, however we also investigated position sensitivity by shifting in three orthogonal directions by ±25mm. Unlike neonates, whose dielectric properties may differ from adults’3, this study on older children considered dielectric properties as age-independent. Thermal modelling using the bioheat equation was also conducted within Sim4Life. These calculations typically use a fixed blood temperature, which approximates patients able to thermoregulate. Since children may have reduced thermoregulation capacity, we also investigated allowing the blood temperature to vary; previous studies4,5 have shown that this can significantly impact the overall modelled temperature changes. Variable blood temperature can be enabled within the Sim4Life thermal solver via the “use body core heating”; we refer to this option as “variable blood temperature”. Temperature dependent perfusion in skin and fat were also modelled as described by Murbach et al6. As with dielectric properties, we used adult-based thermal properties for all models. In each case thermal simulations were run using a power level that would lead to maximum SAR within IEC guidelines7, as calculated in the EM simulations.Results
Figure 1 shows results from EM simulations for all models, including the B1+ within a central axial slice, and projections of the 10g averaged SAR (SAR10g). Figure 2A summarizes these results plotting both the head averaged SAR (hdSAR) and peak spatial SAR10g (psSAR10g) for each model. We observe a small decrease in hdSAR with increasing body mass. For net power normalisation a linear relationship hdSAR (W/kg/W) = 0.23 – 0.0011 M, where M is the body mass, fits the data with R2=0.86. There is no apparent correlation between M and psSAR10g. Figure 2B plots the ratio of psSAR10g to hdSAR for all models. Since the ratio of the IEC limits for these quantities is 3.125 (in normal mode)7, a ratio of observed values below 3.125 means that hdSAR is the limiting quantity: all models are in this regime. HdSAR changes by <5% when models are shifted by ±25mm in 3 orthogonal directions (SI/AP/RL); psSAR10g changes are similarly small for shifts in AP/RL directions but can be up to 50% for SI direction.Figure 3 summarizes thermal simulation results for 60 minutes of exposure. In each case the power level was set such that hdSAR = 3.2W/kg according to the EM simulation results. Figure 3A plots maximum and core temperature as a function of time for each model when variable blood temperature was active. Figure 3B indicates the time at which IEC temperature limits would be exceeded (core temp increase >0.5°C or maximum temp > 39°C); in general the core temperature limits are the first to be exceeded and the time for this to happen is shortest for the smallest models. Figure 4 shows temperature maps for this scenario – it is clear that the average temperatures in the smaller models’ heads are higher after 60 minutes’ exposure. Figure 3C plots temperatures for the case that a fixed blood temperature is used; in this case all models are compliant with the IEC temperature guideline limits.Discussion & Conclusions
Simulations suggest that while psSAR10g is not significantly correlated with subject mass, hdSAR is generally higher in subjects of smaller body mass with an approximately linear dependence. Using the fitted linear relationship, we expect hdSAR to increase by 30% when body mass reduces from 70kg to 30kg. Further reducing the mass from 30kg to 15kg would result in an additional 8.6% increase in hdSAR. This is important because hdSAR is predicted to be the limiting case for all models for the head coil simulated in this work.The standard approach for MRI safety related thermal simulations is to use a fixed blood temperature, which resulted in temperature changes within IEC guideline limits after 60 minutes’ exposure at maximum SAR. If blood temperature is allowed to vary, modelling poor thermoregulation, then the lower body mass models exceed core temperature limits within 60 minutes. This is likely to be conservative since in addition to excluding thermoregulation it does not account for all heat loss mechanisms (e.g. evaporation). Importantly, it also suggests that the main potential risk is systemic, rather than local heating, which can be mitigated by temperature monitoring.Acknowledgements
This work was supported by a Wellcome Trust collaboration in science award [WT201526/Z/16/Z], by core funding from the Wellcome/EPSRC Centre for Medical Engineering [WT203148/Z/16/Z] and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London and/or the NIHR Clinical Research Facility. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. We also acknowledge the receipt of scientific licenses for Sim4Life from Zurich MedTech (www.zurichmedtech.com).References
- Food and Drug Administration. FDA clears first 7T magnetic resonance imaging device. https://www.fda.gov/news-events/press-announcements/fda-clears-first-7T-magnetic-resonance-imaging-device (2017)
- Gosselin, M.-C. et al. Development of a new generation of high-resolution anatomical models for medical device evaluation: the Virtual Population 3.0. Phys. Med. Biol. 59, 5287–5303 (2014).
- Kurtzbard, R., Price, A. N., Hajnal, J. V., Hand, J. W. & Malik, S. J. Evidence for tissue dielectric property differences between neonates and adults: a retrospective study using MR-EPT. in Proc Intl Soc Mag Reson Med 2021 3779 (2021).
- Hirata, A., Asano, T. & Fujiwara, O. FDTD analysis of human body-core temperature elevation due to RF far-field energy prescribed in the ICNIRP guidelines. Phys. Med. Biol. 52, 5013–5023 (2007).
- Hirata, A., Asano, T. & Fujiwara, O. FDTD analysis of body-core temperature elevation in children and adults for whole-body exposure. Phys. Med. Biol. 53, 5223–5238 (2008).
- Murbach, M. et al. Thermal tissue damage model analyzed for different whole-body SAR and scan durations for standard MR body coils. Magn. Reson. Med. 71, 421–431 (2014).
- International Electrotechnical Commission (IEC) 60601-2-33 Medical electrical equipment - particular requirements for the safety of magnetic resonance equipment for medical diagnosis. (2015).
Figures
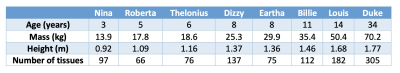
Table 1. Properties of models used in the simulations (from Gosselin et al2)
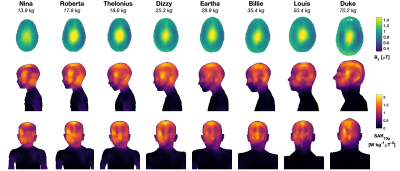
Figure 1: B1+ and SAR10g for all models, depicted at the same spatial scale. Top row: B1+ distributions in central axial plane normalised to have mean B1+ = 1 μT; Middle/bottom rows: SAR10g distributions as maximum projections in sagittal/coronal views, respectively, normalised in the same way as the B1+ maps (i.e. these results give SAR per B1+).
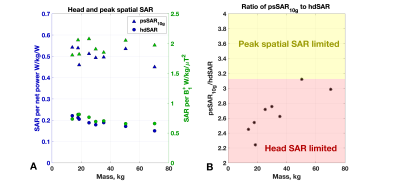
Figure 2: (A) Head average (hdSAR) and peak spatial (psSAR10g) SAR as a function of model mass. Two normalisations commonly used for SAR estimation on commercial scanners are plotted – to net power (i.e. forward-reflected) and to average B1+. (B) Ratio of psSAR10g to hdSAR for each model. A ratio below 3.125 indicates that the limiting value is the hdSAR (IEC limit 3.2 W/kg) as opposed to psSAR10g (IEC normal mode limit 10 W/kg): all models are in this regime.
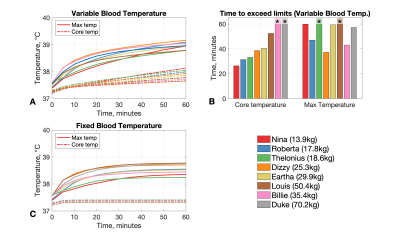
Figure 3: Summary of results from thermal simulations. (A) Temperature data from simulations using variable blood temperatures. In this scenario IEC guidelines for core (change of 0.5°C) and/or maximum local temperature (39°C) are exceeded for most models. (B) Times to exceed IEC guidelines for the data presented in part A. Asterisks indicate the limit was not exceeded. (C) Temperature data for simulation with fixed blood temperature. In this case none exceed the maximum limit of 39°C, and core temperature increases are ~0.1°C for all models.
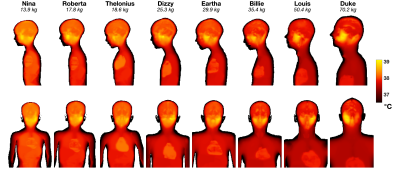
Figure 4: Maximum projections of temperature after 60 minutes at head average SAR = 3.2W/kg for calculations including variable blood temperature. Upper row: sagittal view; Lower row: coronal view.
DOI: https://doi.org/10.58530/2022/2551