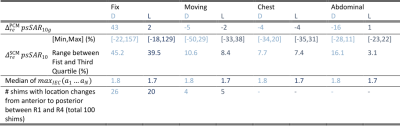2547
Respiratory motion affects peak spatial SAR value and location – impact on safety supervision for 7T cardiac imaging1Physikalisch-Technische Bundesanstalt (PTB), Braunschweig and Berlin, Berlin, Germany, 2Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 3Department for Biomedical Magnetic Resonance, Otto-von-Guericke University Magdeburg, Magdeburg, Germany
Synopsis
Respiratory motion impacts peak spatial specific absorption rate (psSAR) at cardiac UHF MR imaging as shown previously for individual$$$B_1^+$$$shim vectors. Here two saftey supervision modes were compared: a local SAR control mode (SCM) and a channel-wise power control mode (PCM).
Results show amplitude and location changes of the psSAR between inhale and exhale and this effect depends on the coil setup, element type and breathing pattern. Respiratory-induced psSAR variations are lower when applying PCM compared to SCM, while PCM is more conservative with respect to the total power allowed.
Introduction
The impact of respiratory motion on cardiac UHF MR imaging is a major challenge. It limits not only image quality introduced by$$$\text{ }B_1^+\text{ }$$$inhomogneities1–5 but can also introduce electric field variations and, thus, peak spatial specific absorption rate (psSAR) variations during respiration. While this effect has been shown exemplarily for specific shim settings1, it remains yet unclear how this effect impacts scans performed at 7T using different shims given the scanner's safety supervision. For the latter, two different modes are frequently applied by the scanner i) a local SAR-control mode (SCM), often realized by virtual observation points6 or ii) a power-control mode (PCM) that limits the maximum power per channel.For both of these modes we therefore investigate the respiratory impact on local SAR values given$$$\text{ }10^6\text{ }$$$random shims. Variations are compared for different respiration patterns, coil setups and element types in an EM simulation environment. Furthermore, we focus on location changes of psSAR between inhale and exhale.
Methods
A recently proposed1 finite difference time domain EM simulation setup with a 7T 16-TX/RX channel body array7 (8xdipoles/8xloops) and a breathing body model8 for different breathing patterns is applied1,9. For conventional breathing (chest+abdominal) two coil setups are investigated: a Moving setup where the coil moves with a fixed minimum distance between coil and body, and a Fixed setup where the coil does not move while the coil-body-distance varies with respiration. 5 respiratory states from exhale to deep inhale (R1-R5) are implemented for Moving. R5 slightly exceeds the maximum chest displacements found in our own measurements. Only R1-R4 are used for Fixed, and state R4(Moving) equals R4(Fixed). Furthermore, abdominal breathing is simulated with Fixed (denoted as Abdominal(F)) and chest breathing with Moving (denoted as Chest(M)). Dipoles (D) only, loops (L) only and the combination of all 16 elements (LD) are investigated.$$$10^6$$$ random complex phase+amplitude shim vectors $$$U=\left( u_1 e^{i \varphi_1},...,u_Ne^{i\varphi_N} \right)^T$$$ were calculated and applied to the 10g averaged Q-matrix$$$\text{ }\left(Q_{10g}\right)\text{ }$$$resulting in 10g-averaged local SAR:
$$ SAR_{10g}(x) = U^\dagger \cdot Q_{10g}(x) \cdot U.$$
Q-matricies were reduced to virtual observation points6 (VOPs). Measured vectors U and VOPs for the body model are often used in SCM to monitor the peak spatial SAR $$$psSAR_{10g}^{SCM}$$$ during scanning10–12. Alternatively, the channel-wise power can be limited and supervised3,13,14 using PCM. Here, an upper limit of $$$SAR_{10g}$$$ is calculated per voxel using Hölders inequality equation14–16
$$SAR^{PCM}_{10g}:= ||U||_\infty^2\sum^{N,N}_{i,j}|Q_{10g}^{i,j}\left(vox\right)|\geq SAR_{10g}$$
Based on this upper limit an upper peak spatial $$$SAR_{10g}$$$ limit can be calculated that is independent of the applied shim phases:
$$psSAR^{PCM}_{10g}:=max\left(SAR^{PCM}_{10g}\right)\geq pssRA_{10g}$$
U vectors are normalized to achieve $$$psSAR_{10g}$$$ values of 20W/kg (IEC 60601-2-33) for exhale and $$$psSAR_{10g}$$$ variations between exhale (R1) and inhale (R4) are calculated:
$$\Delta_{re}psSAR_{10g}=\frac{psSAR_{10g}\left(R4\right)-psSAR_{10g}\left(R1\right)}{psSAR_{10g}\left(R1\right)}$$
Furthermore, maximum applicable amplitudes for the shims are calculated, with the constraint to stay below 20W/kg (IEC 60601-2-33) for inhale and exhale. Those amplitudes are subsequently referred as $$$u_{IEC}=max\left(u_1...u_N\right)$$$.
Results
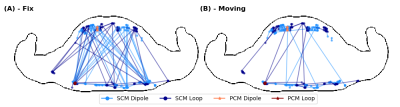
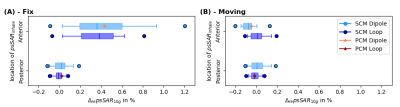
Fig.1A shows animated maximum intensity projections for the Fixed setup for the upper limit when applying PCM-mode. Scaling U such that results in $$$psSAR_{10g}\left(R1\right)=20W/kg$$$ exceeding the limits by 43%. The SCM is sensitive to shim vector phases and may yield in $$$psSAR_{10g}^{SCM}$$$ values changing their location during respiration (Fig.1B). Furthermore, dedicated shims may yield strong respiration-induced variations in $$$psSAR_{10g}^{SCM}$$$ of up to 150% (Fig.1C).The $$$psSAR_{10g}$$$ variations observed for PCM are smaller compared to SCM (Fig.2A-B). Nevertheless, PCM is more conservative, and using SCM 1.8-fold higher shim vector amplitudes $$$u_{IEC}$$$ are applicable(Tab.1). For both modes largest variations are observed for the Fixed setup $$$\left(|\Delta_{re} ^{PCM}psSAR_{10g}|<43\%, |\Delta_{re}^{SCM}psSAR_{10g}|<157\%\right)$$$ and smaller variations ($$$psSAR_{10g}^{PCM}$$$) and narrower distributions ($$$psSAR_{10g}^{SCM}$$$) are seen for loops compared to dipoles (Tab.1). A shift in $$$psSAR_{10g}$$$ locations from anterior to posterior during inhalation (exhale vs. inhale) is observed for all setups, but most for Fix (D/L: 26%/20%) (Fig.3,Tab.1). If $$$psSAR_{10g}^{SCM}$$$ of setup Fix is located anteriorly a broader distribution of $$$\Delta_{re}^{SCM}psSAR_{10g}$$$ is observed compared to shims with $$$psSAR_{10g}^{SCM}$$$ located posteriorly (Fig.4A). For $$$psSAR_{10g}^{PCM}$$$ no respiratory induced anterior/posterior location changes are observed and $$$psSAR_{10g}^{PCM}$$$ is always located anteriorly for the dipole and posteriorly for the loop.
Discussion & Conclusion
This work investigates respiratory induced $$$psSAR_{10g}$$$ amplitude and location variations for multiple breathing patterns of a body model assuming two common safety supervision methods, a SAR-controlled (SCM) and a power-controlled mode (PCM). The results indicate the range of respiratory $$$psSAR_{10g}$$$ variations with an online SCM by up to 150%. In contrast, upper limits for $$$psSAR_{10g}$$$ by the PCM show smaller variation, but lead to smaller applicable amplitudes compared to SCM. Considering the 6min IEC limit, spatial variation of the $$$psSAR_{10g}$$$ over the respiratory cycle is beneficial as it spatially distributes the power deposition over time. Nevertheless, several shim vectors showed little to no location variation of the $$$psSAR_{10g}$$$ which possibly leads to higher $$$psSAR_{10g}$$$ values in free breathing measurements as expected from simulations that include only a single respiratory state. The posterior location of the $$$psSAR_{10g}^{SCM}$$$ for the loops and less respiratory induced motion in this body region, maybe explain smaller respiratory variations in this mode compared to the dipoles. Results for conventional, abdominal and chest breathing are similar, however, multiple body models are needed to infer potential respiration-related safety factors for safety supervision.Acknowledgements
This work was supported by the German Research Foundation (DFG), grant number SCHM 2677/2-1.References
1. Schön N, Petzold J, Schmitter S, et al. Impact of respiration on B1+ field and SAR distribution at 7 T using a novel EM simulation setup. Proc Intl Soc Mag Reson Med. 2020;1120.
2. Padormo F, Larkman DJ. Assessing and Correcting Respiration Induced Variation of B1 in the Liver. Proc Intl Soc Mag Reson Med. 2009;17:754.
3. Schmitter S, Wu X, Ugurbil K, Van De Moortele PF. Design of parallel transmission radiofrequency pulses robust against respiration in cardiac MRI at 7 Tesla. Magn Reson Med. 2015;74:1291-1305.
4. Nehrke K, Börnert P. Free-Breathing Abdominal B1 Mapping at 3T Using the DREAM Approach. Proc Intl Soc Mag Reson Med. 2012;3356.
5. Dietrich S, Aigner CS, Kolbitsch C, et al. 3D Free‐breathing multichannel absolute Mapping in the human body at 7T. Magn Reson Med. 2020;85:2552-2567.
6. Eichfelder G, Gebhardt M. Local specific absorption rate control for parallel transmission by virtual observation points. Magn Reson Med. 2011;66:1468-1476.
7. Ertürk MA, Raaijmakers AJE, Adriany G, Uğurbil K, Metzger GJ. A 16-channel combined loop-dipole transceiver array for 7 Tesla body MRI. Magn Reson Med. 2017;77:884-894.
8. Segars WP, Sturgeon G, Mendonca S, Grimes J, Tsui BMW. 4D XCAT phantom for multimodality imaging research. Med Phys. 2010;37:4902-4915.
9. Schoen N, Seifert F, Metzger GJ, Ittermann B, Schmitter S. Investigation of respiration-induced changes of the scattering matrix by EM simulations and a breathing body model. Proc Intl Soc Mag Reson Med. 2021;3342.
10. Orzada S, Solbach K, Gratz M, et al. A 32-channel parallel transmit system add-on for 7T MRI. PLoS One. 2019;14:1-20.
11. Herrler J, Liebig P, Gumbrecht R, et al. Fast online-customized (FOCUS) parallel transmission pulses: A combination of universal pulses and individual optimization. Magn Reson Med. 2021;85:3140-3153.
12. Gras V, Vignaud A, Amadon A, Le Bihan D, Boulant N. Universal pulses: A new concept for calibration-free parallel transmission. Magn Reson Med. 2017;77:635-643.
13. Metzger GJ, Van De Moortele PF, Akgun C, et al. Performance of external and internal coil configurations for prostate investigations at 7 T. Magn Reson Med. 2010;64:1625-1639.
14. Petzold J, Ittermann B, Frank S. Robustness of pTx safety concepts to varying subjects and subject positions. Proc. Intl. Soc. Mag. Reson. Med. 2021;2488.
15. Hölder O. Ueber einen Mittelwerthabsatz. Nachrichten von derKönigl Gesellschaft der Wissenschaften und der Georg zu Göttingen. 1889;2:38-47.
16. Seifert F, Cassara A, Weidemann G, Ittermann B. Reliable and robust RF safety assessment of transmit array coils at ultrahigh fields. Proc. Intl. Soc. Mag. Reson. Med. 22 2014;4891.
Figures
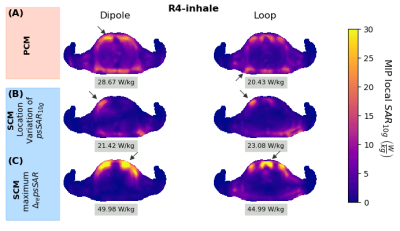
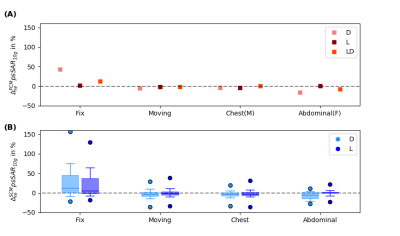
Fig.2: $$$\Delta_{re}psSAR_{10g}$$$ in dependence of setup and element type. Setup: conventional breathing with a Fix and Moving setup, Chest breathing and Abdominal breathing, Element type: dipoles only(D), loops only(L) and loop and dipole(LD). In (A) the variations for the power-controlled mode (PCM) and in (B) the variations of $$$10^6$$$ random amplitude and phase shims using the SAR controlled mode (SCM) are shown. The maximum variations of $$$\Delta_{re}^{PCM}$$$ are a factor 2-9/9-65 (D/L) smaller compared to $$$\Delta_{re}^{SCM}$$$ depending on the setup (Tab.1).