2546
Effects of Intersubject Differences on Scattering Parameters, SAR, and B1+ in a 7T 8ch. Head Coil1Vanderbilt University Institute of Imaging Science, Vanderbilt University, Nashville, TN, United States, 2Department of Physics and Astronomy, Vanderbilt University, Nashville, TN, United States, 3Department of Biomedical Engineering, Vanderbilt University, Nashville, TN, United States, 4Department of Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN, United States
Synopsis
Shorter wavelengths and higher frequencies at UHF contribute to complicated electric field distributions and higher power absorption in the human head leading to potential safety concerns. Scattering parameters, local and global SAR, and B1+ fields are calculated in an 8-channel surface loop Tx array simulated over 70 head-and-shoulder models of 10 tissue compartments. RF shimming methods and pulse sequences are analyzed over each model to demonstrate local and global SAR variation in a population. Patient proximity, coil loading and design, patient composition, and RF shim weights contribute to the variations in SAR and B1+ experienced by each subject.
Introduction
As MRI field strength increases, the wavelengths of radiofrequency (RF) fields required to excite nuclear magnetic resonances decrease causing inhomogeneous excitation and higher power absorption1. Intersubject differences further complicate the fields in the human head and can vary significantly in a population2. Safety factors3, dielectric padding4, universal pulse design5, virtual observation point algorithms6 ,and neural networks7 have all been applied to optimize patient-specific safety and transmission/reception (Tx/Rx) metrics. Simulations demonstrating intersubject electromagnetic field differences in the head have been limited to the 6 virtual family models8. We aim to quantify the range of differences experienced by 70 head models within an 8-channel RF coil tuned and matched for 7T.Methods
MR and CT datasets were used to segment 69 head models into 9 tissue compartments. The models were then affixed to the shoulder and neck portion composed entirely of muscle (the 10th tissue compartment) of the virtual population Duke model9 and placed within an 8 channel Tx surface-loop array to simulate the electromagnetic fields at 298 MHz. The Duke model was included as the 70th head model for a benchmark comparison. The coil was tuned and matched for the median sized head with respect to volume and the same capacitor values were used for every model to mimic a coil in a clinical setting. S-parameters were tracked for each head model. Shim scenarios using the Gerchberg-Saxton (GS) algorithm10, circularly polarized (CP) shim weights, and spokes pulse design over both the entire brain and a select slice of each model were performed. Various specific absorption rate (SAR) metrics and B1+ maps were calculated.Results
The S-parameters over all 70 models (figure 1) vary depending on the coil location and the subject. The coils near the front of the head, coils 1,2,7, and 8 had 3,9,11 and 13 subjects respectively with less than 20% power transmitted at the port. Over a 4mm axial slice in the brain (figure 2), the iterative magnitude least squares (MLS) GS algorithm achieved the lowest mean Coefficient of Variation (CoV) of |B1+| with a value of 15% with a range of 10%. The traditional shims that give a circularly polarized field when unloaded (CPul) achieved a CoV mean of 28% with a range of 16%. A phase-centered shim (CPl) achieved a CoV mean of 40% with a range of 35%. The whole head SAR was well below the 3.2 W kg-1 International Electrotechnical Commission (IEC) limit for each shim scenario for every head, but the peak local SAR10g was well above the normal operating mode IEC limit of 10 W kg-1 for select patients11. In figures 3 and 4, local SAR10g distributions are plotted across their spatial locations and tissue labels. As previously reported the periphery of the patient suffers the highest SAR values with skin suffering higher SAR regardless of shim weights. The worst case local SAR10g occurred on the shoulder of a specific patient (figure 3). Although local SAR limits are exceeded more often than global and head SAR limits, the average SAR in the head in a 6-minute scan (figure 5) is shown to vary per patient depending on the sequence and increases with the number of pulses.Discussion
The S-parameter variation in the coils near the face is an example of the loading due to facial features affecting coil matching. Optimizing the transmit field over the 4mm axial slice without regard for SAR metrics, the utility of the GS algorithm is demonstrated across many subjects. The cost function was only regularized by the weights of each shim squared which is proportional to the power delivered to each coil port. GS had the least number of models who violated the 10 W kg-1 limit. This is a concrete example of RF shimming outperforming the conventional CP shim in transmit homogeneity, global, and local SAR over a population. The concentration of local SAR10g near the nose and back of the head indicate care should be taken to ensure coils near the face are safe no matter the facial feature. Transceive arrays may need to take this into account when receive architectures are designed to be as close to the subject as possible to maximize signal reception. Average SAR increasing with the number of pulses demonstrates the tradeoff between increased resolution at UHF and tissue heating although average global and head SAR may be easily monitored in the bore with pickup coils12 or equivalent circuit models13.Conclusion
The results demonstrate the importance of monitoring local SAR10g for every patient as select individuals may suffer higher peak values due to head anatomy or proximity to the coil. The results further emphasize the need for careful patient positioning within a coil14 and demonstrate a need for discriminating the cause of field variations unless patient-specific safety strategies are implemented within the scanner. Individual patient’s safety using a surface loop-based transmission array is not guaranteed with RF-shimming alone. Local SAR10g is shown to be the limiting safety metric within pulse design over 70 head models. Non-linear pulse design methods may offer improved B1+ homogeneity but must account for their effects on local SAR10g variations.Acknowledgements
This work was supported by NIH grants R21 EB024311 and R01 NS095291.References
1. Nagel AM, Zaiss M, Straub S, et al. Pros and cons of ultra-high-field MRI/MRS for human application. Prog Nucl Magn Reson Spectrosc. 2018;109:1-50.
2. Fiedler TM, Ladd ME, Bitz AK. SAR Simulations & Safety. Neuroimage. 2018;168:33-58.
3. Le Garrec M, Gras V, Hang MF, Ferrand G, Luong M, Boulant N. Probabilistic analysis of the specific absorption rate intersubject variability safety factor in parallel transmission MRI. Magn Reson Med. 2017;78(3):1217-1223.
4. Van Gemert J, Brink W, Webb A, Remis R. High-permittivity pad design tool for 7T neuroimaging and 3T body imaging. Magn Reson Med. 2019;81:3370-3378.
5. Gras V, Vignaud A, Amadon A, Le Bihan D, Boulant N. Universal pulses: A new concept for calibration-free parallel transmission. Magn Reson Med. 2017;77(2):635-643.
6. Eichfelder G, Gebhardt M. Local specific absorption rate control for parallel transmission by virtual observation points. Magn Reson Med. 2011;66(5):1468-1476.
7. Meliadò EF, Raaijmakers AJE, Sbrizzi A, et al. A deep learning method for image-based subject-specific local SAR assessment. Magn Reson Med. 2020;83(2):695-711.
8. De Greef M, Ipek O, Raaijmakers AJE, Crezee J, Van Den Berg CAT. Specific absorption rate intersubject variability in 7T parallel transmit MRI of the head. Magn Reson Med. 2013;69(5):1476-1485.
9. Christ A, Kainz W, Hahn EG, et al. The Virtual Family - Development of surface-based anatomical models of two adults and two children for dosimetric simulations. Phys Med Biol. 2010;55(2).
10. Setsompop K, Wald LL, Alagappan V, Gagoski BA, Adalsteinsson E. Magnitude Least Squares Optimization for Parallel Radio Frequency Excitation Design Demonstrated at 7 Tesla With Eight Channels. Magn Reson Med. 2008;59:908-915.
11. IEC 60601-2-33. IEC. 2015;3.2(06):Medical electrical equipment-Part 2–33.
12. Qian D, El-Sharkawy A-MM, Bottomley PA, Edelstein WA. An RF dosimeter for independent SAR measurement in MRI scanners. Med Phys. 2013;40(12).
13. Jiang W, Yang F, Wang K. Individualized and accurate SAR characterization method based on an equivalent circuit model in MRI system. In: Proceedings of the International Society of Magnetic Resonance in Medicine. ; 2021:2302.
14. Kopanoglu E, Deniz CM, Erturk MA, Wise RG. Specific absorption rate implications of within-scan patient head motion for ultra-high field MRI. Magn Reson Med. 2020;84(5):2724-2738.
Figures
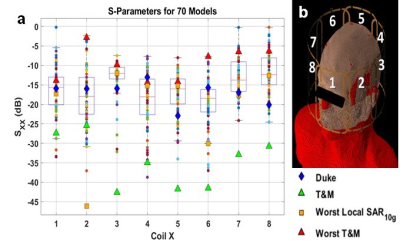
Figure 1: Each coil was simulated individually assuming perfectly decoupled neighboring coils. S11 was tracked to measure the response of each channel to variations in the loading of the head model. The coil was tuned and matched (T&M) to the median-sized head in the database with respect to volume and the S-parameters. The capable range of the S11 values from each subject demonstrates subject to subject variability purely based on the impedance of the load of the subject.
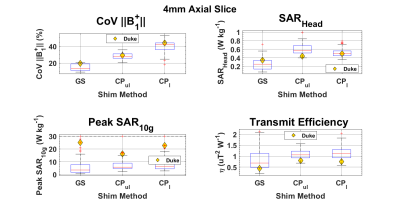
Figure 2: Three RF shimming methods were applied to the parallel Tx array’s field maps across all 70 head models. The field of excitation consisted of a 4mm axial slice in the center of the RF coil. The non-linear GS algorithm outperformed each CP shim condition in field homogeneity, the algorithm was run without regard for SAR limits. Local SAR10g increased for 16 of the 70 models when GS shims were applied versus the conventional circularly polarized weights.
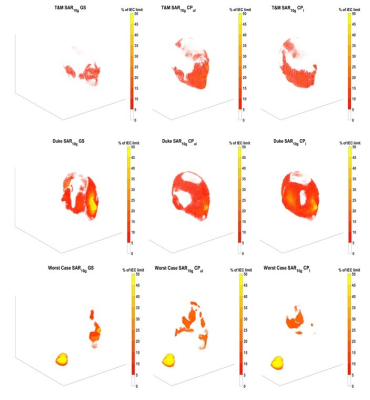
Figure 3: Three specific subjects local SAR10g spatial distributions are plotted with respect to the 10 W kg-1 IEC limit. The T&M case is the head model with ideal frequency and circuit matching (< -25 dB) for every coil. The Duke model is the most anatomically accurate model with 51 tissue compartments. The Worst-Case local SAR10g subject has a localized region on the shoulder that is exposed to over 10 times the IEC limit for each shim condition.
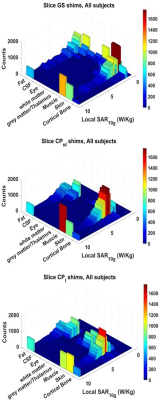
Figure 4: For each shim condition, most of the peak points occur in a skin tissue compartment. It is therefore important in model segmentation to include accurate skin data representation as these tissues are more likely to experience higher local SAR values.
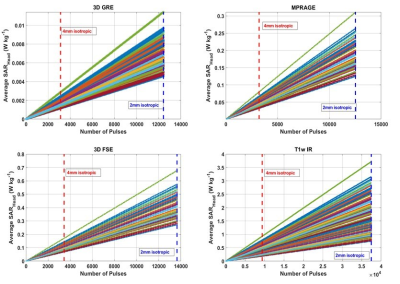
Figure 5: Under Local SAR constraints, heads will reach the IEC average SAR limit at different points given some pulse sequence with N pulses within the 6-minute average window. As the resolution of a scan performed within 6-minutes increases, so does the difference in Average SAR experienced by individual patients.