2540
Time-efficient and reproducible relaxation measurements by 31P-MR fingerprinting in human brain at 7T1CIBM Center for Biomedical Imaging, Lausanne, Switzerland, 2Laboratory for Functional and Metabolic Imaging, EPFL, Lausanne, Switzerland, 3Animal Imaging and Technology, EPFL, Lausanne, Switzerland
Synopsis
In this abstract, we report the reproducibility of the new 31P-MRF technique on healthy volunteers. We show that relaxation and concentration rates can be estimated fast and accurate in good agreement with state-of-the-art methods in the human brain and in phantoms at 7T. This novel efficient technique is able to achieve a 5-fold acquisition time reduction of 31P relaxation parameter measurements, using only 6 min scan time.
Introduction
31P-Magnetic resonance spectroscopy (31P-MRS) is used for measuring non-invasively concentrations of several phosphorus metabolites in human brain. Especially the high-energy phosphates as phosphocreatine (PCr), adenosine triphosphate (ATP) and inorganic phosphate (Pi) are of interest to characterize abnormalities of brain bioenergetics in neurological diseases and other pathological conditions. The knowledge of relaxation times of metabolites is also important for both quantification and investigation of physiological and pathological processes, as their changes reflect alterations in cellular microenvironments. Due to the low sensitivity of 31P-MRS, the measurements of both T1 and T2 relaxation times are time consuming. Recently, we established a novel 31P - magnetic resonance fingerprinting (31P-MRF) for human brain applications at 7T for the simultaneous and fast measurement of concentrations and relaxation times [1]. The technique is based on the quantitative and time-efficient MRF framework by Ma et al [2]. In a preclinical study, it was already proven to be promising to measure multi-parameters in a short period of time [3]. In this study, we investigate the reproducibility of this new technique and compare it with conventional relaxation measurements.Methods
A balanced steady-state free precession type sequence was developed and implemented. The repetition time (TR) was fixed to 18.9 ms and a Shinar-le-Roux slice selective excitation pulse train (2 cm slice thickness) with varying flip angles (FA) was applied. To increase T1 sensitivity a broadband inversion pulse (HS4; 10.24ms; inversion bandwidth = 4.9kHz; B1/2rt = 1kHz) is applied. The FA pattern follows after a 10ms inversion delay. It consists mainly out of two parts: 1) 518 FAs in a sinusoidal pattern interrupted by another broadband inversion pulse and 2) 181 FAs of drastic changes. The echo time was fixed and set so that the center of ADC is at the center of TR. Figure 1 briefly described the MRF scheme used in this study. A Gradient echo B0 map (30 mm slice; 6 s) was acquired afterwards to incorporate B0 inhomogeneities into the dictionary.MR experiments were performed on a 7T/68cm MR scanner (Siemens Medical Solutions, Erlangen, Germany) with a 1H quadrature surface coil (10cm-diameter) and a single-loop 31P coil (7cm-diameter) designed for data acquisition from the occipital lobe. 7 phantoms with 100 mM Pi and different concentrations of MnCl2 and Gadovist were scanned with different γB1+ to test the 31P-MRF sequence. Inversion recovery (IR) method and sSPECIAL sequence based multi-TE method were used to acquire T1 and T2 relaxation times for the phantom and to compare with the results of MRF scheme. Data was also acquired on 6 healthy subject (3 female; 3 males; age 19-27 years) who provided written informed consent. To examine the reproducibility of the 31P-MRF protocol, IR (TI=60, 200, 400, 670, 1590, 3000 ms; TR=4 sec & no inversion; TR=4, 20 sec) method and multi-TE method (TE=15, 20, 27, 35, 48, 70, 100, 200 ms; TR=7.5 sec) were repeated 2 times per subject in pseudorandom manner with a 15 min break in between the scans. The number of averages were chosen to have the same scan time of 26 min for the MRS-FP (83 averages + 1 dummy scan), IR (16+1 scans & 6+1 for TR=20sec) and multi-TE (8/12/16/16/16/16/16/16+2 scans) protocol.
Results
Table 1 displays the results of the phantom experiment. The 31P-MRF is able to estimate relaxation times robustly in the presents of B1+ inhomogeneities, introduced by biological tissues and surface coil properties. Figure 2 shows the mean and standard deviation of T1 and T2 estimations of 31P-MRF for different metabolites compared to the state-of-the-art methods. The estimations are in good agreement and bias free even for low number of averages. The mean test-retest reproducibility is validated in Figure 3 using the coefficient of variation (CV). For a scan time of 6 min, the CV of the estimations are still in a good range.Discussion
We showed a fast and accurate relaxation parameter estimation for the main 31P metabolites using 31P-MRF. This new quantification method allows us to decrease the scan time by 5 times to 6min. Especially T1 can be estimated with an ultra-short estimation time of 2 min. However, T1 relaxation times were estimated robustly, the T2 relaxation times suffer from effects of the long acquisition time and signal losses introduced by the b-SSFP bandpass. The estimations could benefit from a shorter acquisition time, in terms of less susceptibility to motions. Furthermore, an optimization of the TR is necessary to avoid stopband filtering effect on the main metabolites especially for Pi, which is strongly influenced by the b-SSFP filter in the current setup.Acknowledgements
This work was supported by the Swiss National Science Foundation (grants n° 320030_189064). We acknowledge access to the facilities and expertise of the CIBM Center for Biomedical Imaging, a Swiss research center of excellence founded and supported by Lausanne University Hospital (CHUV), University of Lausanne (UNIL), Ecole polytechnique fédérale de Lausanne (EPFL), University of Geneva (UNIGE) and Geneva University Hospitals (HUG).
References
[1] Song-I Lim, Mark Stephan Widmaier, Yun Jiang, and Lijing Xin. Rapid T1, T2 measurements and SNR evaluation by 31P MR fingerprinting in human brain at 7T. ISMRM 2021; Poster 1531.
[2] Ma D, Gulani V, Seiberlich N, et al. Magnetic resonance fingerprinting. Nature 2013; 495:187–192 doi: 10.1038/nature11971.
[3] Wang CY, Liu Y, Huang S, Griswold MA, Seiberlich N, Yu X. 31 P magnetic resonance fingerprinting for rapid quantification of creatine kinase reaction rate in vivo. NMR Biomed. 2017; 30:1–14 doi: 10.1002/nbm.3786.
Figures
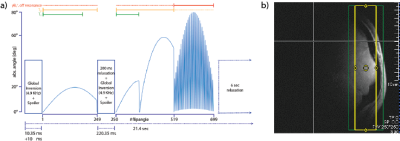
Figure 1: (a) The 31P-MRF FA scheme and (b) exemplary 2cm slice selection in the occipital lob.
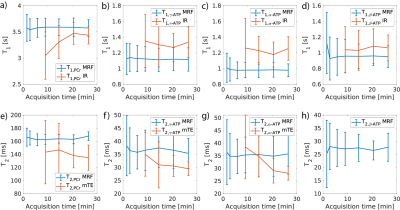
Figure 2: Mean T1 relaxation times with STD measured by the 31P-MRF and the IR method over different acquisition durations for (a) PCr, (b) γ-ATP, (c) α-ATP, (d) β-ATP and mean T2 relaxation times with STD measured by the 31P-MRF and the multi-TE (mTE) method over different acquisition durations for (e) PCr, (f) γ-ATP, (g) α-ATP, (h) β-ATP.
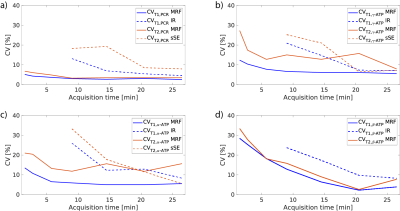
Figure 3: CV [%] over acquisition time of 31P-MRF, IR and sSE for (a) PCr, (b) γ-ATP, (c) α-ATP, (d) β-ATP.
Table 1: 31P-MRF phantom experiments in comparison with IR and multi-TE (mTE) method. The 31P-MRF is able to estimate T1 and T2 accurately for different γB1+.