2517
Learning-based prediction of encoding capability for acquisition schedule of Magnetization Transfer Contrast MR fingerprinting1School of Electrical Engineering, Korea Advanced Institute of Science and Technology, Daejeon, Korea, Republic of, 2Division of MR Research, Department of Radiology, Johns Hopkins University, Baltimore, MD, United States, 3F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States
Synopsis
Magnetization transfer contrast MR fingerprinting (MTC-MRF) is used to quantify multiple tissue parameters of free bulk water and semisolid macromolecule using pseudo-randomized MRF schedules. An optimal design of the MRF schedule is important to improve the quantification accuracy and reduce the scan time. However, lack of the objective function that represents the encoding capability of MRF schedule hinders the reliable optimization. In this study, we propose a novel metric that represents the encoding capability of MRF schedule based on recurrent neural network. Unlike the conventional metrics based on indirect measurements, the proposed learning-based metric directly measures the tissue quantification errors.
Introduction
Magnetization transfer contrast MR fingerprinting (MTC-MRF) is used to quantify multiple tissue parameters of free bulk water and semisolid macromolecule using pseudo-randomized MRF schedules1-2. A design of the MRF schedule is essential for the quantification accuracy and the scan time, and optimizing the acquisition schedule has been of recent interest in the MRF field3-7. However, it has been challenging due to lack of a reliable metric for the encoding capability of MRF sequences. Most of the current metrics are based on indirect measurements, such as the signal discrimination power between different tissue parameters and the SNR efficiency based on Cramer-Rao lower bound (CRLB) of MR signal models. The aforementioned indirect metrics cannot completely agree with the quantification errors. Although the Monte Carlo methods accurately measures the quantification errors, the high computational cost remains to be a challenge. In this study, we propose a fast novel metric that represents the encoding capability of MRF schedule using deep recurrent neural network. In particular, the learning-based metric directly represents tissue quantification errors for the encoding capability.Methods
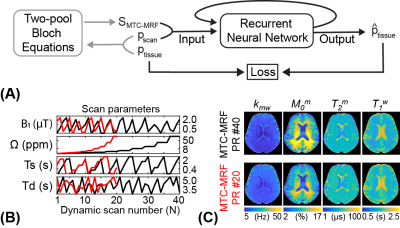
The proposed learning-based metric uses the trained RNN model to represent the encoding capability of MRF schedules. Therefore, the training of the neural network should precede the metric calculation.Network Training
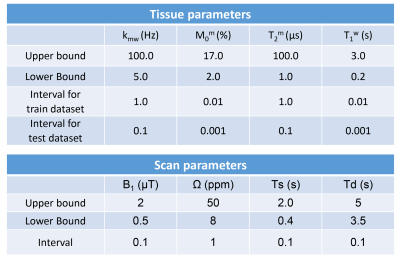
A recurrent neural network (RNN) was designed to quantify water and MTC parameters from MR fingerprints regardless of their MRF schedules. The RNN framework consists of 1) a generation of MRF schedules with scan parameters pscan = [B1, Ω, Ts, Td, N], 2) a simulation of MTC-MRF signals using Bloch equations with the generated MRF schedule and the given tissue parameters ptissue = [Kmw, Mom, T2m, T1w], and 3) a MTC quantification from the simulated MTC-MRF signals (Fig. 1). To train the RNN model, 40 million combinations of tissue and scan parameters were chosen. Particularly, each scan parameter was randomly selected within the pre-defined range (Table 1) so that various patterns and lengths of MRF schedules could be considered.
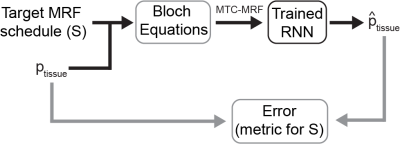
Predicting the Encoding Capability
MTC-MRF signals are simulated through Bloch equations with a given target MRF schedule (S) and the test dataset consisted of ten thousand tissue parameters (ptissue), and fed to the trained RNN for the tissue parameter quantification (Fig. 2). The proposed metric, for predicting the encoding capability of the MRF schedule S, is defined by calculating normalized root mean square errors (nRMSE) between the estimated tissue parameters and the ground-truth ($$$\mathrm{\hat{p}_{tissue}}$$$ ).
Validation
To validate the proposed learning-based metric, two thousand MRF schedules of ten dynamics scans were retrospectively constructed from the pseudo-random (PR) schedule of 40 dynamic scans and used to assess the encoding capability. For each schedule, the agreement between the calculated metric and the estimated in vivo tissue parameter maps were evaluated. For in vivo studies, six healthy volunteers (four females and two males; age range: 27-41 years; mean age: 36 years) were scanned on a 3T MRI scanner after written informed consent was obtained with the approval of the institutional review board.
Results
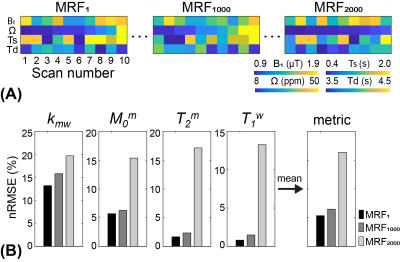
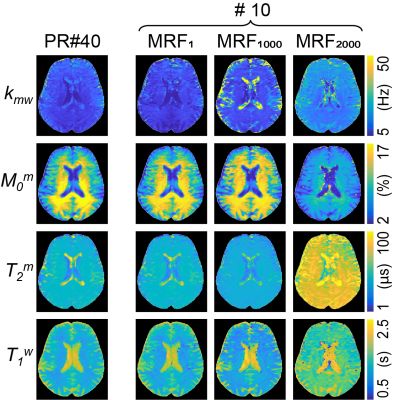
Two thousand MRF schedules of ten dynamic scans were sorted according to the proposed learning-based metric as shown in Fig. 3A. The average nRMSE values of four tissue parameters (Kmw, Mom, T2m, and T1w) were calculated with the trained RNN model and converted to the proposed metric (Fig. 3B). The calculation of the proposed metric takes 2.2 sec for each schedule with ten thousand tissue parameter sets (0.7 sec for MTC-MRF signal simulation and 1.5 sec for RNN), hence total 1.2 hour for two thousand schedules. Three representative MRF schedules were chosen that showed the smallest, middle, and largest values of the proposed metric (MRF1, MRF1000, and MRF2000, respectively) and used to quantify the water and MTC parameter maps of a healthy volunteer human brain. The parameter maps estimated from the three schedules were compared to those from the PR schedule of 40 dynamic scans. The estimated parameter maps from MRF1 were in excellent agreement with those from the PR #40 (Fig. 4). However, less agreements were observed for MRF1000 and MRF2000 schedules. The Pearson correlation coefficient values between the PR #40 and MRF1, MRF1000 and MRF2000, respectively were 0.75, 0.54, and 0.28 for kmw, 0.97, 0.94, and 0.80 for M0m, 0.92, 0.87, and 0.63 for T2m, and 0.93, 0.88, and 0.45 for T1w (p<0.05 for all). The excellent agreement was observed between the calculated metrics using the simulated data and in vivo results.Discussion & Conclusion
We proposed a novel metric that predicts the encoding capability of MRF schedule based on the deep recurrent neural network. Unlike the conventional metrics based on indirect measurements, the proposed learning-based metric directly measures tissue quantification errors. The proposed metric is also computationally efficient due to its simple feed-forward deployments of RNN in test phase (2.2 sec for each schedule). In addition, any MRF schedule is applicable for the proposed metric because RNN model was trained to handle various patterns and lengths of MRF schedule, providing a great flexibility for input schedules. The proposed learning-based metric could be a fast reliable metric for MRF pulse sequence design.Acknowledgements
This work was supported by the Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety) (Project Number: 1711138003, KMDF-RnD KMDF PR 20200901 0041-2021- 02), and by grants from the Rad BriteStar Award from the Department of Radiology Johns Hopkins University School of Medicine, the National Institutes of Health (R01NS112242).References
1. Kim B, Schar M, Park H, Heo HY. A deep learning approach for magnetization transfer contrast MR fingerprinting and chemical exchange saturation transfer imaging. Neuroimage 221: 117165 (2020).
2. Kang B, Kim B, Schar M, Park H, Heo HY. Unsupervised Learning for Magnetization Transfer Contrast MR Fingerprinting (MTC-MRF): Application to Chemical Exchange Saturation Transfer (CEST) and Nuclear Overhauser Enhancement (NOE) Imaging. Magn Reson Med 85: 2040-2054 (2021).
3. Sommer K, Amthor T, Doneva M, Koken P, Meineke J, Börnet P. Towards predicting the encoding capability of MR fingerprinting sequences. Magn Reson Imaging 41: 7-14 (2017).
4. Cohen O, Rosen MS. Algorithm comparison for schedule optimization in MR fingerprinting. Magn Reson Imaging 41: 15-21 (2017).
5. Bo Z, Haldar JP, Congyu L, Dan M, Yun J, Griswold MA, Setsompop K, Wald LL. Optimal Experiment Design for Magnetic Resonance Fingerprinting: Cramer-Rao Bound Meets Spin Dynamics. IEEE Trans Med Imaging 38: 844-861 (2019).
6. Perlman O, Herz K, Zaiss M, Cohen O, Rosen MS, Farrar CT. CEST MR-Fingerprinting: Practical considerations and insights for acquisition schedule design and improved reconstruction. Magn Reson Med 83: 462-478 (2020).
7. Jordan SP, et al. Automated design of pulse sequences for magnetic resonance fingerprinting using physics-inspired optimization. Proceedings of the National Academy of Sciences 118: 40 (2021).
Figures