2513
Combining Region-optimized virtual coils with low rank reconstructions for accelerated cardiac MR Fingerprinting1King's College London, London, United Kingdom
Synopsis
Cardiac Magnetic Resonance Fingerprinting (MRF) produces co-registered, multi-parametric maps from highly accelerated acquisitions. Low rank methods leveraging temporal information have enabled highly undersampled MRF reconstructions. However, residual aliasing from these reconstructions can potentially propagate through the MRF framework into the parametric maps. Region-Optimized Virtual coils have recently been proposed for undersampled reconstructions in conventional imaging, by suppressing unwanted sources of aliasing signal. Here we combine both methods and investigate its performance in Cardiac Magnetic Resonance Fingerprinting.
INTRODUCTION:
Cardiac Magnetic Resonance Fingerprinting (MRF)1,2 has been investigated for myocardial tissue characterization. In 2D, breath-hold acquisitions are commonly employed to minimize respiratory motion, limiting the available scan time to the duration of a feasible breath-hold. Additionally, ECG-triggering used to minimize cardiac motion reduces scan efficiency, and consequently cardiac MRF requires highly accelerated acquisitions. Low rank models3,4,5 have been deployed in MRF to cope with the acceleration requirements. MRF requires the residual artefacts to be incoherent, otherwise they may propagate into the parametric maps. Recently, Region-Optimized Virtual coils (ROVir)6 have been proposed to suppress unwanted signal in the FOV, which may otherwise contribute to residual aliasing, demonstrating improved performance in conventional Cartesian undersampled imaging. In cardiac MRF, subcutaneous fat (or other strong signal sources) can introduce coherent artefacts and potentially introduce errors in the maps. Here, we combine ROVir with a Low Rank Inversion (LRI) and investigate its feasibility for cardiac MRF.METHODS:
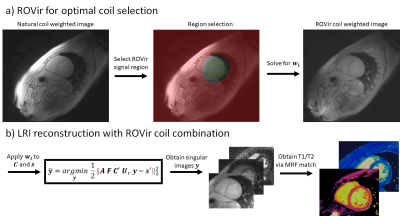
The proposed ROVir+LRI framework integrates the optimal coil combination from ROVir into the subspace constrained LRI reconstruction for cardiac MRF (Fig. 1). First, a ROI is select around the myocardium, marking the “signal” region $$$ A $$$; the complementary region in the FOV is considered as “interference” region $$$ B $$$. According to the ROVir theory6, there is a set of linear weight vectors that maximizes the signal-to-interference ratio: $$$ SIR(w_i) = \frac{w_i^HAw_i}{w_i^HBw_i} $$$. The vectors $$$ w_i $$$ can be found by solving $$$Aw_i = \lambda Bw_i$$$ via a generalized eigenvalue decomposition. Once found, $$$ w_i $$$ can be applied to the coil sensitivities and k-space data to suppress signal from region $$$ B $$$, while maintaining signal from region $$$ A $$$. These “virtual” coils and k-space data can then be used in a subspace constrained reconstruction3,4,5. Here, we follow the LRI notation, where the set of singular images $$$ y $$$ (compressing the temporal contrast dynamics) is obtained by solving: $$ \hat{y} = \mathit{argmin}_y \left \| AFC' U_r y - s' \right \| _2 ^2 $$, where $$$ A $$$ is the sampling trajectory, $$$F$$$ is the Fourier transform, $$$C'$$$ are “virtual” ROVir coil sensitivities, $$$ Ur $$$ are the left singular vectors following a singular value decomposition of the MRF dictionary and $$$s'$$$ are “virtual” ROVir k-space data. Following the reconstruction, the MRF parametric maps may be obtained by standard template matching of $$$y$$$ to the MRF dictionary.EXPERIMENTS:
Eight healthy subjects were scanned at a 1.5T scanner (Philips Ingenia) using a cardiac MRF sequence for simultaneous T1/T2 mapping. The MRF sequence was ECG-triggered, employing Inversion Recovery (for T1) and T2 preparation pulses (for T2) as described in previous work.7 Imaging parameters included field of view (FOV) = 315x315 mm2; 8 mm slice thickness; resolution = 1.75x1.75 mm2; TE/TR = 0.9/7.1 ms; gradient echo readout; 6-10º sinusoidally varying flip angle; 1080 time-points; nominal scan time 18s. Acquired data was reconstructed with LRI and with ROVir+LRIRESULTS:
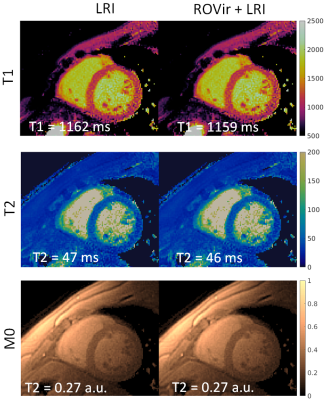
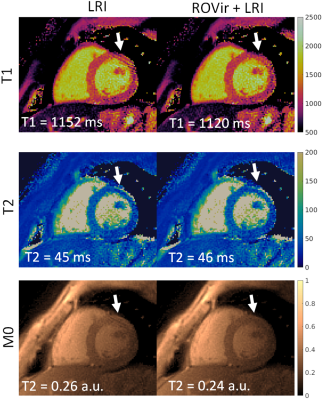
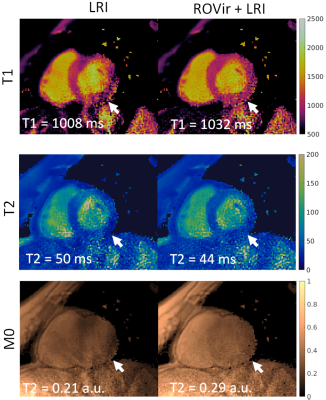
A comparison of LRI and ROVir+LRI revealed that in the majority of cases (6/8), both methods produced virtually the same results (Fig. 2). There were two cases were residual artefacts were present in the T1/T2 LRI maps, but were reduced in T1/T2 ROVir+LRI maps (Fig. 3 and Fig. 4). In both cases, the residual coherent aliasing was due to strong signal sources (e.g. off-resonant excitations and/or fat) in the edges of the FOV.CONCLUSION:
The feasibility of Region-Optimized Virtual coils plus Low Rank Inversion was investigated for cardiac MR Fingerprinting. Preliminary results indicate similar performance in most cases, however in some situations ROVir+LRI can reduce coherent aliasing and improve map quality. Future work will compare this approach with other methods of coil selection.Acknowledgements
This work was supported by EPSRC (EP/P001009, EP/P032311/1, EP/P007619/1) and Wellcome EPSRC Centre for Medical Engineering (NS/ A000049/1).References
1. Hamilton JI, Jiang Y, Chen Y, et al. MR fingerprinting for rapid quantification of myocardial T 1 , T 2 , and proton spin density. MRM 2017;77:1446–1458.
2. Jaubert O, Cruz G, Bustin A, Schneider T, Botnar RM, Prieto C. Dixon-cMRF : cardiac tissue characterization using three-point Dixon MR fingerprinting. 2019:5–7 doi: 10.1002/mrm.26216.2.
3. McGivney DF, Pierre E, Ma D, et al. SVD compression for magnetic resonance fingerprinting in the time domain. IEEE Trans. Med. Imaging 2014;33:2311–2322 doi: 10.1109/TMI.2014.2337321.
4. Zhao B, Setsompop K, Adalsteinsson E, et al. Improved magnetic resonance fingerprinting reconstruction with low-rank and subspace modeling. Magn. Reson. Med. 2018;79:933–942 doi: 10.1002/mrm.26701.
5. Assländer J, Cloos MA, Knoll F, Sodickson DK, Hennig J, Lattanzi R. Low rank alternating direction method of multipliers reconstruction for MR fingerprinting. Magn. Reson. Med. 2018;79:83–96 doi: 10.1002/mrm.26639.
6. Kim D, Cauley SF, Nayak KS, Leahy RM, Haldar JP. Region‐optimized virtual (ROVir) coils: Localization and/or suppression of spatial regions using sensor‐domain beamforming. Magnetic Resonance in Medicine. 2021 Jul;86(1):197-212.
7. Cruz G, Qi H, Jaubert O, Kuestner T, Schneider T, Botnar RM, Prieto C. Generalized low‐rank nonrigid motion‐corrected reconstruction for MR fingerprinting. Magnetic Resonance in Medicine. 2021 Oct 2.
Figures