2505
Application of APTw imaging in clear cell renal cell carcinoma1Xi'an Gaoxin Hospital, Xi'an, China, 2Philips Healthcare, Beijing, China
Synopsis
The application of amide proton transfer (APT) weighted imaging in molecular imaging of craniocerebral tumors has been relatively mature, but rarely reports on renal tumors. This study conducted a preliminary comparative analysis of clear cell renal cell carcinoma (ccRCC) and healthy adult renal APT imaging. The APTw values 6.40(6.26~7.75)% of tumor foci in the ccRCC group was significantly higher than that of healthy adult renal (2.05±0.24) %The preliminary results indicate that APT technology has potential clinical application value in the diagnosis of ccRCC.
The application of amide proton transfer (APT) weighted imaging in molecular imaging of craniocerebral tumors has been relatively mature, but rarely reports on renal tumors. This study conducted a preliminary comparative analysis of clear cell renal cell carcinoma (ccRCC) and healthy adult renal APT imaging. The APTw values 6.40(6.26~7.75)% of tumor foci in the ccRCC group was significantly higher than that of healthy adult renal (2.05±0.24) %.The preliminary results indicate that APT technology has potential clinical application value in the diagnosis of ccRCC.
Introduction:
As a new non-invasive MRI molecular imaging technology, amide proton transfer (APT)-weighted (APTw) imaging can indirectly reflect the proliferation and protein metabolism of tissues and lesions from the perspective of metabolism. It has been initially applied in the diagnosis of urinary diseases, such as prostate cancer [1], cervical cancer [2] and ovarian disease [3]. Due to technical reasons, there are few reports on its application in renal diseases. Based on the good reproducibility of APTw imaging of the renal of healthy adults in the early stage, this study preliminarily explored the application value of APTw imaging in renal tumors.
Methods:
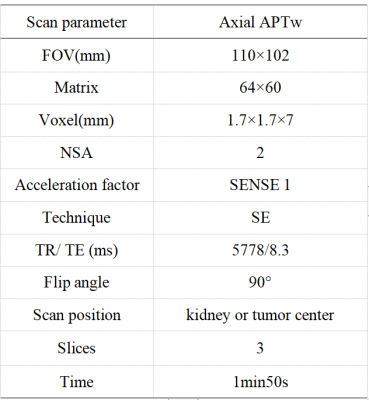
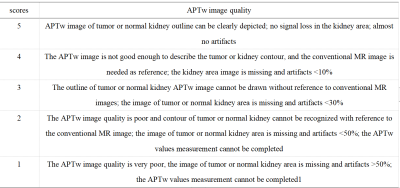
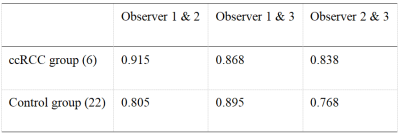
Seven cases (age range from 34 to 66 years, 48.8 ±12.4, 5 males) with pathologically confirmed ccRCC were included as the ccRCC group; 27 healthy volunteers (age range from 24 to 46 years, 30±8.9, 10 males) served as a control group. Both the ccRCC group and the control group underwent intermittent end-respiratory breath-holding renal 3D-APTw imaging on a 3.0T MR scanner (Ingenia CX, Philips Healthcare). APTw imaging was performed using a saturation pulse with duration of 2s and strength of 2µT. According to the Likert Scale scoring method for APTw image quality evaluation (Table 1), lower scores (1 or 2) were not included in the APTw values measurement. APTw images were analyzed independently by three radiologists with 3-, 6- and 10-years’ experience, blinded to each other's measurements. Three regions of interest (ROI) were placed on the right kidney in APTw image of ccRCC or control group (Table 1). All ROIs avoid blood vessels and artifacts. A ROI was manually placed by each observer on the location of the lesion in ccRCC group or random measurement of right kidney in control group according to the APTw imaging, and its APTw value was measured. The intraclass correlation coefficient (ICC) was used to evaluate the consistency of the three observers. A non-parametric test was used to compare the difference of APTw values between the ccRCC group and the control group.
Results:
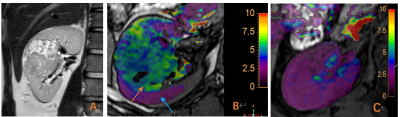
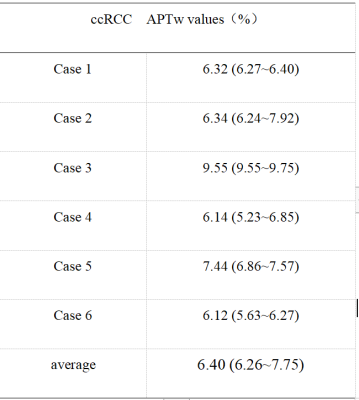
According to Likert scale scoring result, 6 cases were excluded due to a small lesion and poor image quality (1 in ccRCC group and 5 in control group). APTw values measured in the ccRCC group and control group by three observers were well consistent (ICC>0.75) (Table 3 and Table 4). The APTw values measured for the ccRCC group 6.40 (6.26~7.75) % were significantly higher than that of control group (2.05±0.24) % (Figure 1), and the difference was statistically significant (z=-3.675, P<0.001) (Table 4).
Discussion:
ccRCC is the most common malignant tumor of the kidney. The tumor cell density is high, which increases the production of protein and polypeptide substances, accelerates the rate of water proton-amide proton transfer, and increases the APTw value [4]. As the degree of tumor malignancy increases, the nuclear atypia increases, which can induce the interaction between the macromolecular D sub-substances and the hydrophobic cell membrane, thereby promoting the release of proteins and peptides and causing the tumor APTw value to increase [5]. In addition, studies have reported that the proteins and peptides released in the process of tissue necrosis of malignant tumors also promote the rate of amide proton transfer [6]. ccRCC is prone to hyaloid degeneration and cystic necrosis. Among the 6 cases of ccRCC in this group, there are four cases had definite necrosis of tumor tissue. It is speculated that the above reasons may be the reason for the higher APTw values of this group of tumor tissues, and it is necessary to continuously increase the inclusion of the ccRCC group for in-depth analysis.
Conclusion:
Preliminary results show the feasibility of APTw imaging of intermittent end-respiratory breath-holding in healthy adult kidney and ccRCC. At the same time, it is found that the APTw value of ccRCC is significantly higher than that of normal renal parenchyma. Therefore, APTw imaging has potential clinical value in the diagnosis of renal tumors.
Acknowledgements
NoReferences
1.Jia G, Abaza R, Williams JD, et al. Amide proton transfer MR imaging of prostate cancer: a preliminary study[J]. J Magneason Imaging, 2011, 33(3): 647-654. DOI:10.1002/jmri.22480.
2. Meng N, Wang X, Sun J, et al. Application of the amide proton transfer-weighted imaging and diffusion kurtosis imaging in the study of cervical cancer[J]. Eur Radiol, 2020, 30(10): 5758-5767.
3. Ishimatsu K, Nishie A, Takayama Y, et al. Amide proton transfer imaging for differentiating benign ovarian cystic lesions: Potential of first time right[J]. European Journal of Radiology, 2019, 120: 108656.
4. Nishie A, Takayama Y, Asayama Y, et al. Amide proton transfer imaging can predict tumor grade in rectal cancer[J]. Magneason Imaging, 2018, 51: 96-103.
5.Tang Y, Dundamadappa SK, Thangasamy S, et al. Correlation of apparent diffusion coefficient with Ki-67 proliferation index in grading meningioma[J]. AJR Am J Roentgenol, 2014, 202(6): 1303-1308.
6.Togao O, Hiwatashi A, Yamashita K, et al. Grading diffuse gliomas without intense contrast enhancement by amide proton transfer MR imaging: comparisons with diffusion- and perfusion-weighted imaging[J]. Eur Radiol, 2017, 27(2): 578-588.
Figures