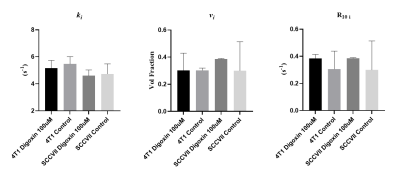2504
Estimation of cellular water exchange rate, intracellular volume fraction and longitudinal relaxation time in cancer cells1Radiology, Weill Cornell Medical College, New York, NY, United States
Synopsis
The T1 recovery curves of cells may be sensitized to water exchange when suspended in solution with gadolinium-based contrast agent. The non-monoexponential recovery curves are fitted to a two-site exchange model to estimate the water exchange rate. This estimation process requires the inclusion of relevant parameters such as volume mol fraction and intracellular relaxation time, cannot be accurately estimated from a single curve. Here, we propose a multiple curve fitting approach, which infuses the model with additional information. By modelling multiple recovery curves of samples in a titration of GBCAs, we demonstrate an accurate and robust method for measuring invitro water exchange, volume mol fractions and intracellular relaxation rate.
The T1 recovery curves of cells may be sensitized to water exchange across the cell membrane when suspended in solution with gadolinium-based contrast agent. The two-compartment model fitting requires the inclusion of relevant parameters such as volume mol fraction and intracellular relaxation time but cannot be accurately estimated from a single curve. Here, we propose a multiple curve fitting approach, which infuses the model with additional information. By modelling multiple recovery curves of samples in a titration of GBCAs, we demonstrate an accurate and robust method for measuring invitro water exchange, cell volume and intracellular relaxation rate.
Introduction
Cellular water exchange has been shown to be a promising biomarker for cellular energy turnover, with important implications in cancer imaging.1 The T1 recovery of cells may be sensitized to water exchange by increasing R1 in extracellular space with the addition of gadolinium-based contrast agents (GBCAs) and fitting the resultant non-monoexponential recovery with a two-site exchange model (2SX)2. This approach generally requires fixing physiologically relevant parameters, such as intracellular longitudinal relaxation rate and intracellular volume fraction, to estimate water exchange. Here, we propose a method to increase the accuracy of water exchange measurements while also estimating intracellular relaxation rate and intracellular volume fractions by using cells suspended in a titration of GBCA concentrations.Methods
We generated a numerical simulation of the T1 saturation recovery (SR) curves for cells suspended in GBCA at various concentrations (0mM, 1mM, 1.5mM, 2mM) under realistic noise with a Rician distribution. SR curves were generated using the two-site exchange model (2SX)2. Various combinations of simulated SR curves with different concentrations of GBCA were then used to estimate the intracellular relaxation rate (R10i), cellular volume fractions (vi and vex), and water exchange rate from the intracellular to extracellular compartment (ki). In the fitting procedure, the extracellular relaxation rate (R1i = .260 s-1), the equilibrium magnetization (M0 = 1 as curves are normalized to final delay point) and relaxivity of Gadobutrol at 500MHz and 37oC (r1 = 2.89 s-1mM-1) were determined experimentally and fixed (data not shown). The SR curves simulated without GBCA were initially fit with the fast exchange limit (FXL) condition2 to determine R10i. The SR curves simulated with different GBCA concentrations were simultaneously fit with the 2SX, with a multi start optimization approach, to determine ki and vex.Cell lines used in the study included murine tumor lines 4T1 and SCCVII. Cells were grown to confluence in Dulbecco’s Modified Eagle Media supplemented with 10% FBS. Cells were detached with 0.05% Trypsin-EDTA, washed with PBS, and resuspended in four equal aliquots with different concentrations of Gadobutrol (0mM, 1mM, 1.5mM, 2mM) with 10% D2O in PBS as a lock reagent. Cells were concentrated to 5e6 cells per 150μL and added to 1.7mm NMR tubes. The NMR tubes were centrifuged for 5 minutes at 300g and 4oC and the supernatant was removed. The samples were kept at 4oC until scanned.
Water proton R1 was measured with a SR pulse sequence, with 28-30 sequential delay times, at 11.7T and 37oC on a Bruker Avance III HD 500 MHz spectrometer with a TCI 1H-19F/13C/15N triple resonance cryogenic probe (Bruker Instruments). The recovery curves were fit with the 2SX model as described previously. Samples prepared from the same cell population, with different concentrations of GBCA, were scanned consecutively.
To explore the influence of metabolic activity on cellular water exchange, we conducted a drug challenge with Na+/K+-ATPase selective inhibitor Digoxin. Toxicity on cell lines were determine with a CCK-8 assay. Cells were grown to confluence and subsequently incubated with 100μM Digoxin or a vehicle control for 24 hours prior to scanning.
Results
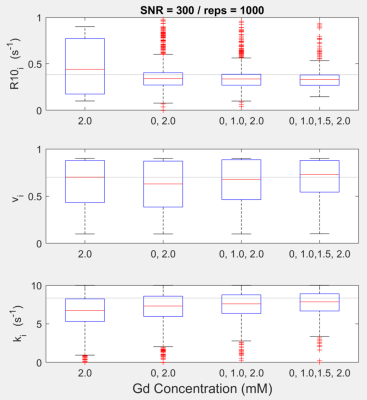
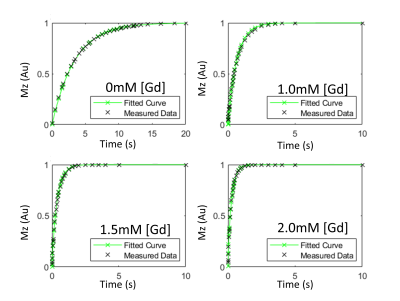
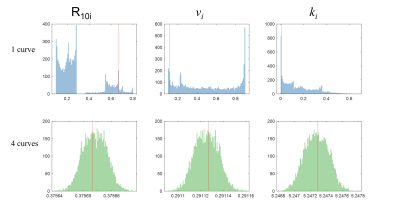
The numerical simulation demonstrated that increasing the number of curves fitted simultaneously reduces variability in intracellular relaxation rate estimation (R10 i) and accuracy of the exchange rate constant (ki) as the median approaches closer to the ground truth with the addition of more curves with different GBCA concentrations (Figure 1).For the in vitro experiment, the SR curves from 4T1 cells, with a titration of GBCA, demonstrate a reasonable fit from the proposed simultaneous multiple curve fitting approach (Figure 2). The simultaneous fitting with multiple curves converges on a single solution compared to a single curve fit that had wide ranges of local minima (Figure 3). The inclusion of more SR curves in the parameter estimation reduces the interquartile range for the water exchange parameter (ki), volume fraction (vi) and, to a lesser extent, the intracellular relaxation rate (R10 i) for 4T1 cells (n=9)(Figure 4).
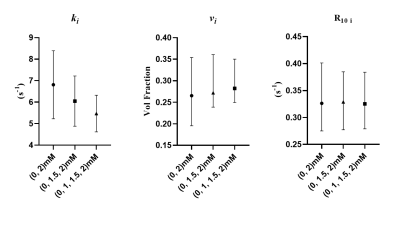
4T1 and SCCVII cells challenged with Na+/K+-ATPase inhibitor Digoxin demonstrated a reduction in the ki and an increase in the intracellular relaxation rate estimation (R10 i) compared to a vehicle control group (Figure 5).
Discussion
We have demonstrated that the proposed approach with multiple GBCA concentrations was able to achieve more accurate and robust estimation of cellular water exchange rates, cellular volume mol fractions and intracellular relaxation time via simulation study and in-vitro experiments. Furthermore, we have demonstrated that the measured water exchange rate is sensitive to cellular metabolic activity, as the inhibition of Na+/K+-ATPase resulted in a reduction in ki.The preliminary finding of this study is in agreement with the Active Contrast Encoding (ACE)-MRI method3 which leverages multiple flip angles to give different weightings of the water exchange effect in the washout data of a DCE MRI scan. In a similar fashion, by infusing more information into the measurement using multiple GBCA concentrations and though simultaneous fitting of those multiple SR curves, we were able to increase accuracy and precision of the estimated parameters.
Some limitations in our experiment include the tradeoff from scanning at a high magnetic field strength. While advantageous for improving SNR, increased T2* effect restricted the use of higher concentrations of GBCA.
Acknowledgements
NIH R01CA160620, R01CA219964, UH3CA228699, NMR core is supported by WCM and NIH shared instrumentation grants S10 OD 016320 and S10 OD028556References
1. Springer, C. S., Li, X., Tudorica, L. A., Oh, K. Y., Roy, N., Chui, S. Y. C., Naik, A. M., Holtorf, M. L., Afzal, A., Rooney, W. D., & Huang, W. Intratumor mapping of intracellular water lifetime: Metabolic images of breast cancer? NMR in Biomedicine. 2014;27(7), 760–773.
2. Gianolio E, Ferrauto G, Di Gregorio E, Aime S. Re-evaluation of the water exchange lifetime value across red blood cell membrane. Biochim Biophys Acta - Biomembr. 2016;1858(4):627-631. doi:10.1016/j.bbamem.2015.12.029
3. Zhang J, Kim SG. Estimation of cellular-interstitial water exchange in dynamic contrast enhanced MRI using two flip angles. NMR Biomed. 2019;32(11):1-17. doi:10.1002/nbm.4135
Figures