2497
Relative cerebral blood volume of tumor habitats can differentiate presurgical IDH-wildtype glioblastoma from IDH-mutant astrocytoma grade 4.1Biomedical Data Science Laboratory. ITACA, Universitat Politècnica de València, Valencia, Spain, 2Oslo University Hospital, Oslo, Norway, 3Institut Catala Oncologia Badalona, Barcelona, Spain, 4Institut de Diagnostic per la Image (IDI), Hospital Dr Josep Trueta, Girona, Spain, 5Instituto de Investigación Sanitaria La Fe, Valencia, Spain, 6Hospital de la Ribera, Alzira, Valencia, Spain, 7Hospital Clinic de Barcelona, Barcelona, Spain, 8void.space Lab, Facultat de Medicina, Universitat de Vic, Vic, Spain
Synopsis
IDH-wildtype glioblastoma and IDH-mutant astrocytoma are classified as different gliomas according to WHO 2021. IDH mutations are key at clinical level, since they are associated with patient prognosis and seem to be critical for treatment selection. Despite these evidences, current protocols do not include the full sequencing for all tumors. In this sense, non-invasive and automatically calculated MRI-based biomarkers can be helpful for the clinical practice.
Our results show that perfusion markers obtained in an automated, repeatable, and non-invasive manner may be candidates for being surrogate predictive markers of IDH mutation status in astrocytomas grade 4.
Introduction
The World Health Organization (WHO) of CNS tumor classification and grading 2021 [1] considers IDH-wildtype glioblastoma and IDH-mutant astrocytoma grade 4 as different tumors, with a prevalence of 95% and 5%, respectively [1, 2]. The mutation status of IDH genes (IDH1 and IDH2) is critical at the clinical level since it is associated with patient prognosis [3-6], associated with a median overall survival (OS) of 15 months for IDH-wildtype glioblastoma versus a median OS of 20 months for patients with IDH-mutant astrocytomas [7]. Furthermore, IDH mutation status is important for treatment planning, including selection of immunotherapies [7], as well as for novel inhibitors targeting mutated IDH proteins [8-9]. Therefore, early-stage stratification of patients with gliomas based on IDH mutation status would facilitate a more accurate prognosis and may also improve the patients treatmen decision making process.Despite its essential role, the complete IDH evaluation can differ according to patient age and clinical guidelines. Considering that protocols do not include a full sequencing for every tumor (recommended for patients older than 55 years only), the use of additional markers could be helpful. In this sense, MRI-based methodologies could have a relevant role, the use of additional, non-invasive MRI biomarkers is warranted, especially at the presurgical stage.
In 2020, Hao Wu et al. [10] evaluated the potential clinical impact of the Hemodynamic Tissue Signature (HTS) method in IDH mutation prediction in patients with gliomas. They found a significantly decreased rCBV for the IDH-mutant group, concluding that the HTS method was proven to have high prediction capabilities for IDH mutation status in high-grade glioma patients. Despite the great interest of these results, the study only included 25 patients with astrocytomas grade 4, of which 9 had mutated IDH.
Purposes
To confirm the clinical value of perfusion-based MRI biomarkers, calculated using the HTS method, to help classify patients with gliomas grade 4 from the presurgical stage. The specific objectives were: 1) To analyze the association between the perfusion biomarkers calculated at tumor and edema habitats with IDH mutation status; 2) to study differences in vascularity between IDH-wildtype glioblastoma and IDH-mutant astrocytoma grade 4, and 3) to analyze survival differences between these two astrocytoma types.Material and Methods
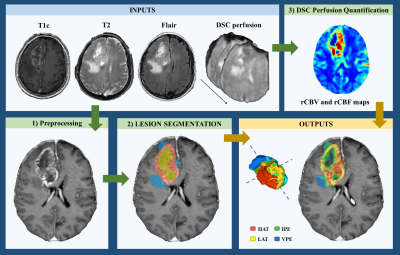
We analyzed clinical and pre-surgical MRI data from 299 retrospective patients with glioma grade 4, including 5% of the entire cohort with mutated IDH (IDHmut) (16 patients). The remaining 283 presented IDH-wildtype glioblastomas. The study cohort included data from the following public datasets: TCGA-GBM (n = 35; 1 IDHmut), Ivy GAP (n = 19, 2 IDHmut) and CPTAC-3 (n = 10; 3 IDHmut), two multicenter studies (NCT03439332, n = 108; 6 IDHmut; and GLIOCAT database [14], n = 107; 3 IDHmut) and a cohort of 20 patients (1 IDHmut) from Hospital La Fe, Valencia, Spain.We used the HTS method [11-13], a fully automated, reproducible, and validated technology, freely available for research purposes at the ONCOhabitats platform (www.oncohabitats.upv.es), to calculate the relative cerebral blood volume (rCBV) and flow (rCBF) of vascular habitats. These habitats can be described as specific regions within the tumor and edema with distinct vascular properties), including the high angiogenic tumor habitat (HAT), the low angiogenic tumor habitat (LAT), the infiltrated peripheral edema (IPE) and the vasogenic peripheral edema (VPE). Figure 1 summarizes the four stages of the HTS method.
We performed Uniparametric Cox regression analysis to analyze the association of rCBV and rCBF of each habitat with tumor IDH mutation status. In addition, a Mann-Whitney U-test was used to asses any vascular differences between gliomas with different IDH mutation status.
Results
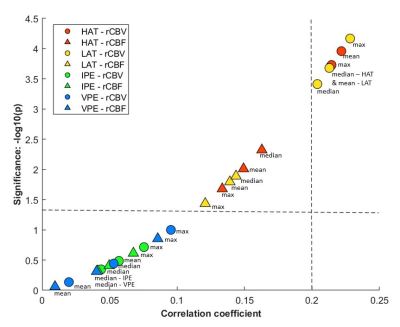
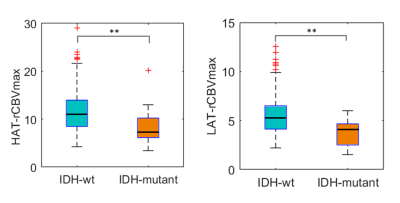
Mean, median, and maximum rCBV calculated at HAT and LAT yielded coefficients higher than 0.2 when anlalyzing the correlation with IDH mutation status (p<0.05) (Figure 2). We selected rCBVmax calculated at HAT and LAT for the following analysis since they yielded the best results.Differences in rCBVmax at HAT and LAT between IDH-mutant astrocytomas and IDH-wildtype glioblastoma were analyzed (Table 1, Figure 3). For both habitats, the median, and the minimum and maximum rCBVmax were higher for the IDH-wildtype glioblastoma group (p<0.001).
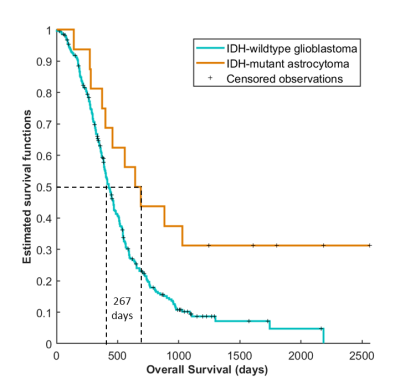
To ensure the clinical interest of differentiating between IDH-wildtype and IDH-mutant, we carried out the Kaplan Meier analysis (Figure 4) and log-rank test. We found significant survival differences (p = 0.00141) between the IDH-mutant astrocytoma grade 4 group and the IDH-wildtype glioblastoma group, with median survival rates of 627 and 400 days respectively.
Discussion and Conclusions
With this study, we have demonstrated the clinical relevance of the perfusion MRI biomarkers calculated using the HTS method to identify astrocytoma grade 4. In particular, lower rCBV values calculated at the high- and low- angiogenic tumor habitats were significantly associated with IDH-mutant astrocytoma grade 4.Current clinical guidelines for patients with glioma do not include sequencing for all patients. Moreover, sequencing requires collecting a tumor sample by biopsy or during surgery with added patient burden. Our results show that MRI-based perfusion biomarkers using an automated, repeatable, and non-invasive approach may be potential surrogate predictive tool for identifying IDH mutation status in patients with astrocytomas grade 4.
Acknowledgements
M.A.T was supported by DPI2016-80054-R (Programa Estatal de Promoción del Talento y su Empleabilidad en I+D+i).This work was partially supported by the ALBATROSS project (National Plan for Scientific and Technical Research and Innovation 2017-2020, No. PID2019-104978RB-I00) (JMGG); H2020-SC1-2016-CNECT Project (No. 727560) (JMGG), and H2020-SC1-BHC-2018-2020 (No. 825750) (JMGG). EFG was supported by the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No 844646 and South-Eastern Norway Regional Health Authority Grant 2021057. This study was partially funded by the Fundació La Marató TV3 (665/C/2013) (http://www.ccma.cat/tv3/marato/projectes-financats/2012/231/).
References
[1]: Louis DN, Perry A, Wesseling P et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro-Oncology 2021;23(8),1231-1251
[2]: Louis N, Perry A, Reifenberge RG et al. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol 2016;131:808
[3]: Ohgaki H, Kleihues P (2013) The definition of primary and secondary glioblastoma. Clin Cancer Res 19:764–772. doi:10.1158/1078-0432.CCR-12-3002
[4]: Mirchia K and Richardson TE. Beyond IDH-Mutation: Emerging Molecular Diagnostic and Prognostic Features in Adult Diffuse Gliomas. Cancers 2020;12(7):1817
[5]: Yan H., Parsons D.W., Jin G et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 2009;360:765–773.
[6]: Christians A, Adel-Horowski A, Banan R et al. The prognostic role of IDH mutations in homogeneously treated patients with anaplastic astrocytomas and glioblastomas. Acta Neuro. Comm. 2019; 7(156)
[7]: Han S, Liu Y, Cai SJ, et al. IDH mutation in glioma: molecular mechanisms and potential therapeutic targets. Nature 2020;122,1580-1589
[8]: Kaminska B, Czapski B, Guzik R et al. Consequences of IDH1/2 Mutations in Gliomas and an Assessment of Inhibitors Targeting Mutated IDH Proteins. Molecules 2019;24(5):968
[9]: Popovici-Muller, J., Lemieux, R. M., Artin, E. et al. Discovery of AG-120 (Ivosidenib): a first-in-class mutant IDH1 inhibitor for the treatment of IDH1 mutant cancers. ACS Med. Chem. Lett. 2018; 9,300–305
[10]: Wu H, Tong H, Du X et al. Vascular habitat analysis based on dynamic susceptibility contrast perfusion MRI predicts IDH mutation status and prognosis in high-grade gliomas. European Radiology 2020; doi.org/10.1007/s00330-020-06702-2
[11]: Juan-Albarracín J, Fuster-García E, Pérez-Girbés, et al. Glioblastoma: Vascular habitats detected at preoperative dynamic susceptibility weighted contrast-enhanced perfusion MR imaging predict survival. Radiology 2018; 287:944–954
[12]: Juan-Albarracín, J, Fuster-García E, García-Ferrando GA et al. ONCOhabitats: A system for glioblastoma heterogeneity assessment through MRI. International journal of medical informatics 2019; 128: 53-61
[13]: Álvarez-Torres M, Juan-Albarracín J, Fuster-Garcia E, et al. Robust association between vascular habitats and patient prognosis in glioblastoma: An international multicenter study. Journal of Magnetic Resonance Imaging 2020; 51(5)
[14]: Pineda E, Esteve-Codina A, Martinez-Garcia M et al. Glioblastoma gene expression subtypes and correlation with clinical, molecular and immunohistochemical characteristics in a homogenously treated cohort: GLIOCAT project. Journal of Clinical Oncology 2019; 37(15)
Figures