2494
Stiffening of renal transplants after donation assessed by MR elastography – physiological adaption from donor to recipient1Radiology, Charité – Universitätsmedizin Berlin, Berlin, Germany, 2Urology, Charité – Universitätsmedizin Berlin, Berlin, Germany, 3Medical Informatics, Charité – Universitätsmedizin Berlin, Berlin, Germany
Synopsis
Renal stiffness was investigated by using magnetic resonance elastography (MRE) with tomoelastography-postprocessing in 24 participants (12 renal donor-recipient couples) to explore the influence of kidney transplantation on renal viscoelasticity for the first time. Renal stiffness increases immediately after transplantation. These early changes could be due to changes in renal perfusion and increase in renal metabolism, which is also reflected by increase in renal volume. Cold ischemia time was found in being a contributor to changes of solid renal structures. Inccrease in renal stiffness after transplantation could be a novel predictive biomarker for better renal outcome.
Purpose:
To investigate the influence of kidney transplantation on renal viscoelasticity by multifrequency magnetic resonance elastography (MRE) and thus providing a physiological postoperative baseline for the mechanical response of the transplanted kidney in the host environment.Background:
Kidney transplantation is currently the only treatment that restores kidney function in patients with terminal renal failure[1]. The major challenge in the clinical management of kidney recipients in the immediate posttransplant phase is the timely recognition of acute allograft rejection[2,3]. Early detection of acute rejection is essential for rapid initiation of immunosuppressive shock therapy, which prevents graft loss and improves overall outcome[4]. Currently, noninvasive diagnostic methods are not specific enough and therefore cannot replace invasive biopsies yet[4,5]. Diagnostic tests for assessing the structural-functional integrity of kidney grafts during post-transplant follow-up are needed. Both physiological and pathological changes are associated with changes in renal stiffness, which was demonstrated by multifrequency MRE[6-10]. There is an increasing clinical need to improve noninvasive kidney allograft monitoring and detection of acute allograft rejection. MRE has never been applied to in vivo kidneys prior and post kidney transplantation with the aim of detecting transplant-related biophysical parameter changes, which serves as baseline for clinical studies in the future.Methods:
In this prospective study, 24 study participants (12 kidney donors, 51±9 years, 8 female; 12 kidney recipients, 48±17 years, 2 female) were examined by multifrequency MRE and tomoelastography data processing [10,11] at four vibration frequencies from 40–70 Hz by using air-pressurized drivers. All experiments were conducted on a 1.5-T MRI scanner equipped with a 12-channel phased array surface coil. Shear wave speed (SWS) was given in m/s (as a surrogate of stiffness). All imaging protocols were executed in a paracoronal slice orientation covering the entire kidney. Regions of interest (ROI) were placed covering the entire renal parenchyma excluding the renal pelvis. The same kidney was analyzed in the donor before transplantation and then in the recipient after transplantation. Pre- and postoperative renal viscoelastic changes were analyzed by paired t-test. The relationship between SWS and clinical parameters was tested by Pearson r correlation coefficient.Results:
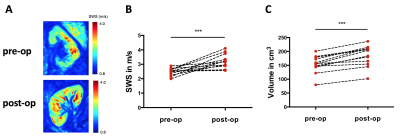
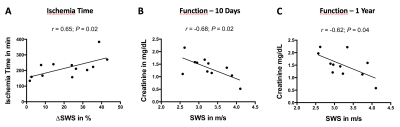
Figure 1A shows representative maps of multifrequency MRE of the same kidney before (pre-op in the donor) and after transplantation (post-op in the recipient). Kidney SWS (pre-op, 2.48±0.25 m/s; post-op, 3.23±0.48 m/s; p<0.001; Fig. 1B) and volume (pre-op, 154±31 mm3; post-op, 185±38 mm3; p<0.001; Fig 1C) increased after transplantation. Cold ischemia time during renal allograft transplantation was positively correlated with relative change in SWS (r=0.65; p=0.02; Fig 2A). Serological creatinine was negatively correlated with SWS after 10 days (r=-0.68; p=0.02; Fig 2B) and after a one-year follow-up (r=-0.62; p=0.04; Fig 2C).Discussion:
In this study we identified physiological changes in viscoelasticity after renal allograft transplantation providing new reference values for clinical studies in the future. We hypothesize that increase in renal stiffness is based on three major contributors: i) changes in perfusion[12], ii) cold ischemia time[13], and iii) increased metabolism[14]. Changes in perfusion result from unphysiological anastomosis of the renal artery and vein to the main iliac vessels as well as denervation of the corresponding renal nerves, which are crucial for regulation of vessel constriction[12]. Cold ischemia time has been identified as a risk factor for cell death and allograft outcome[13]. Changes of these solid tissue structures could be represented by increase in renal stiffness. Increase in renal volume is associated with cellular hypertrophy representing an increased metabolism[14]. These adaptions might be understood as a physiological compensation of renal function, which was completed by two kidneys before donation. Interestingly, renal stiffness was negatively correlated with serological creatinine, a surrogate of renal function. We hypothesize that higher renal stiffness after transplantation might be a predictive marker for better renal allograft outcome.Conclusion:
Multifrequency MRE is sensitive to changes in renal viscoelasticity after allograft transplantation. This novel quantitative and non-invasive imaging biomarker has a high potential for the use in the clinical assessment of allograft rejection in the future.Acknowledgements
The authors would like to thank the German Research Foundation (DFG) for their financial support (project number 467843609, GRK2260 BIOQIC, SFB 1340).References
1. Chadban, S.J.; Ahn, C.; Axelrod, D.A.; Foster, B.J.; Kasiske, B.L.; Kher, V.; Kumar, D.; Oberbauer, R.; Pascual, J.; Pilmore, H.L., et al. Kdigo clinical practice guideline on the evaluation and management of candidates for kidney transplantation. Transplantation 2020, 104, S11-s103.
2. Tierie, E.L.; Roodnat, J.I.; Dor, F. Systematic surgical assessment of deceased-donor kidneys as a predictor of short-term transplant outcomes. European surgical research. Europaische chirurgische Forschung. Recherches chirurgicales europeennes 2019, 60, 97-105.
3. Wang, J.H.; Skeans, M.A.; Israni, A.K. Current status of kidney transplant outcomes: Dying to survive. Advances in chronic kidney disease 2016, 23, 281-286.
4. Kdigo clinical practice guideline for the care of kidney transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2009, 9 Suppl 3, S1-155.
5. Ketteler, M.; Block, G.A.; Evenepoel, P.; Fukagawa, M.; Herzog, C.A.; McCann, L.; Moe, S.M.; Shroff, R.; Tonelli, M.A.; Toussaint, N.D., et al. Executive summary of the 2017 kdigo chronic kidney disease-mineral and bone disorder (ckd-mbd) guideline update: What's changed and why it matters. Kidney international 2017, 92, 26-36.
6. Lang, S.T.; J., G.; A., B.; M., D.; J., B.; B., H.; I., S.; R., M.G.S. Multiparametric quantitative mri for the detection of iga nephropathy using tomoelastography, dwi, and bold imaging. Investigative Radiology 2019, accepted on 24th April 2019.
7. Marticorena Garcia, S.R.; Althoff, C.E.; Dürr, M.; Halleck, F.; Budde, K.; Grittner, U.; Burkhardt, C.; Jöhrens, K.; Braun, J.; Fischer, T., et al. Tomoelastography for longitudinal monitoring of viscoelasticity changes in the liver and in renal allografts after direct-acting antiviral treatment in 15 kidney transplant recipients with chronic hcv infection. Journal of clinical medicine 2021, 10.
8. Marticorena Garcia, S.R.; Fischer, T.; Durr, M.; Gultekin, E.; Braun, J.; Sack, I.; Guo, J. Multifrequency magnetic resonance elastography for the assessment of renal allograft function. Invest Radiol 2016, 51, 591-595.
9. Marticorena Garcia, S.R.; Grossmann, M.; Bruns, A.; Durr, M.; Tzschatzsch, H.; Hamm, B.; Braun, J.; Sack, I.; Guo, J. Tomoelastography paired with t2* magnetic resonance imaging detects lupus nephritis with normal renal function. Invest Radiol 2019, 54, 89-97.
10. Marticorena Garcia, S.R.; Grossmann, M.; Lang, S.T.; Tzschatzsch, H.; Dittmann, F.; Hamm, B.; Braun, J.; Guo, J.; Sack, I. Tomoelastography of the native kidney: Regional variation and physiological effects on in vivo renal stiffness. Magn Reson Med 2018, 79, 2126-2134.
11. Tzschatzsch, H.; Guo, J.; Dittmann, F.; Hirsch, S.; Barnhill, E.; Johrens, K.; Braun, J.; Sack, I. Tomoelastography by multifrequency wave number recovery from time-harmonic propagating shear waves. Medical image analysis 2016, 30, 1-10.
12. Ardalan, M.R.; Tarzamni, M.K. Renal allograft hemodynamic and diameter changes after living donor transplantation. Transplantation proceedings 2006, 38, 388-389.
13. Gorayeb-Polacchini, F.S.; Caldas, H.C.; Fernandes-Charpiot, I.M.M.; Ferreira-Baptista, M.A.S.; Gauch, C.R.; Abbud-Filho, M. Impact of cold ischemia time on kidney transplant: A mate kidney analysis. Transplantation proceedings 2020, 52, 1269-1271.
14. Halleck, F.; Diederichs, G.; Koehlitz, T.; Slowinski, T.; Engelken, F.; Liefeldt, L.; Friedersdorff, F.; Fuller, T.F.; Magheli, A.; Neumayer, H.H., et al. Volume matters: Ct-based renal cortex volume measurement in the evaluation of living kidney donors. Transplant international : official journal of the European Society for Organ Transplantation 2013, 26, 1208-1216.
Figures