2492
Parametric T1 MR Microscopy Detects Gadolinium Residuals in Ex Vivo Rat Kidneys1Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrueck Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany, Berlin, Germany, 2Institute of Vegetative Physiology, Charité – Universitätsmedizin Berlin, Berlin, Germany, Berlin, Germany, 3Experimental and Clinical Research Center, a joint cooperation between the Charité Medical Faculty and the MAX Delbrück Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany, Berlin, Germany
Synopsis
Accumulation of gadolinium (Gd) following use of Gd-based contrast agents (GBCA) is a concern. To study the potential of T1 microscopy for assessing Gd residues in the kidney, rats were administered 8 intravenous doses of various GBCAs over a period of two weeks. Five days following the last administration, the kidneys were collected and fixed in formalin. High resolution T1 maps obtained from ex vivo scans showed significantly reduced T1 levels for both linear and macrocyclic GBCAs. This demonstrates to potential for quantitative T1 mapping to detect Gd residues non-invasively in renal tissue.
Introduction:
More than 30 million enhanced MRI scans are performed annually using Gadolinium (Gd)-based contrast agents (GBCAs) and this number continues to increase1. Detection of Gd residues in certain brain regions and in the skin, even after considerable intervals following GBCA administration, or retention in physiological tissues like brain, bone, liver and kidney etc. triggered safety concerns. Clinical observation, clinical and preclinical MR studies, as well as postmortem histological studies, have indicated much lower residual GBCA levels following administration of macrocyclic GBCA versus the less stable linear agents2. Recent studies in healthy rats with high cumulative dosages of GBCA indicated that residual Gd levels in the kidneys may be two orders of magnitude higher that in the brain. To determine potential toxicological consequences of this deposition, and to investigate the fate of linear and macrocyclic GBCAs and of Gd in the body, further research is required to inform clinical use of GBCAs. This requires, noninvasive assessment of residual GBCAs levels in the kidney. Recognizing this opportunity, this work uses parametric MR microscopy to examine GBCA-induced T1-shortening in ex-vivo rat kidney.Methods:
Eight male Wistar rats (mass 242-303 g, Charles River, Sulzfeld, Germany) were studied. All investigations were approved by LaGeSo of Berlin accordance with the German Animal Protection Law. The experiments were carried out in accordance with the approved guidelines. Rats were kept under standard condition with food and water ad libitum. Rats were randomly allocated to eight groups: six group were exposed to GBCA administration at a dosage regimen of eight intravenous administration of GBCAs for 4 consecutive days per week over a period of two weeks resulting in a cumulative dose of 4.8 mmol Gd/kg bodymass. Three macrocyclic GBCA were studied: 1) gadobutrol (Gadovist; Bayer Vital, Leverkusen, Germany), 2) gadoterate meglumine (Dotarem; Guerbet, Sulzbach, Germany), 3) gadoteridol (ProHance, Bracco Imaging, Konstanz, Germany). The Three linear GBCA were studied: 1) gadobenate dimeglumine (MultiHance; Bracco Imaging), 2) gadodiamide (Omniscan; GE Healthcare Buchler & Co, Braunschweig, Germany), and 3) gadopentetate dimeglumine (Magnevist; Bayer Vital). Two rats served as controls: one received isotonic saline; the other was unmanipulated. Five days after the last injection, rats were sacrificed by exsanguination under anesthesia with 1,5% isoflurane, and the kidneys were removed and fixed in 4% formalin. MRI data were acquired with a 2-channel volumetric transceiver RF coil on a 9.4T animal MR scanner (PharmaScan, Bruker BioSpin). T1 mappings was performed with a RARE technique: TE=9.87 ms, TR array = 100, 170, 250, 336, 435, 550, 685, 848, 1060, 1353, 1843 and 3750 ms, RARE factor=2, slice thickness=0.4 mm, FOV=24×24 mm, matrix = 480×480, resolution 50x50 μm, number of averages=9 and total acquisition time=9h 48m 41 sec for each T1 map. Quantitative visualization, segmentation and validations were done using in-house developed software base on MATLAB and ImageJ.Results:
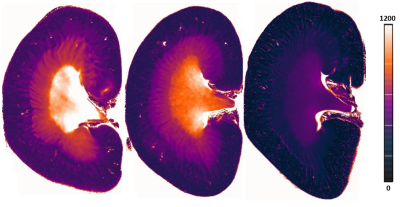
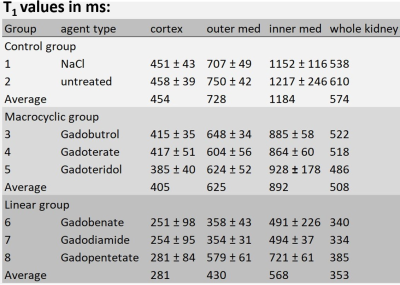
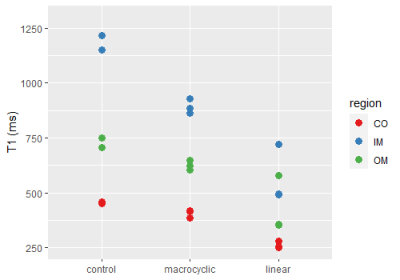
Figure 1 illustrates representative high resolution T1 maps of ex- vivo rat kidneys obtained for the control group, for Gadoteridol administration and for Gadobenate administration. Upon GBCA administration T1-shortening is very well depicted. Table 1 provided a synopsis of renal T1 obtained for the control group and for the rats administrated with GBCA. The control group had T1 relaxation times (mean±standard deviation) of T1=454±41 ms (cortex), T1=728±45 ms (outer medulla) and T1=1184±181 ms (inner medulla). Upon administration of linear GBCA significant T1-shortening was detected in the kidney. Averaged over all linear GBCAs T1 was of T1=284±95 ms (cortex), T1=430±43 ms (outer medulla) and T1=568±37 ms (inner medulla). Upon administration of macrocyclic GBCA T1 was shortened for all three renal layers. An average T1 of 405±40 ms (cortex), 625±52 ms (outer medulla) and 892±60 ms (inner medulla) was found. The most pronounced shortening among the macrocyclic group was observed for gadoteridol (cortex: T1 =385±40 ms, outer medulla: T1=624±52 ms and, inner medulla T1=928±178 ms). The examination of the measurement reproducibility, comparing the first and second examination, yielded and R2= 0.989.Discussion:
Parametric T1 MR microscopy of ex vivo rat kidney demonstrates that residual Gd is detectable five days after the last GBCA injection. T1 shortening obtained for linear GBCAs (ΔT1=-39%) was more pronounced than for macrocyclic GBCA (ΔT1=-11%). Validation of initial results using a second independent measurement demonstrated excellent reproducibility.Conclusion:
Parametric T1 MR microscopy is clinically meaningful for research into the safety and toxicity of GBCA administration. For quantitative assessment of residual GBCA presence in renal tissue with parametric T1 MR microscopy, calibration with quantitative measurements is required. These include but are not limited to plasma mass spectrometry. Histological evaluation of GBCAs in renal tissue is also warranted to detail the toxicological, pharmacokinetic and clinical consequences of these findings obtained from parametric T1 MR microscopy.Acknowledgements
This work was funded in part (TN, ES, SW, TG, KC) by the German Research Foundation (Gefoerdert durch die Deutsche Forschungsgemeinschaft (DFG), Projektnummer 394046635, SFB 1365, RENOPROTECTION). We wish to thank A. Anger for outstanding support. We also thank the MDC-Weizmann Helmholtz International Research School for Imaging and Data Science from the NAno to the MESo (iNAMES).References
[1] Lohrke, Jessica, et al. "25 years of contrast-enhanced MRI: developments, current challenges and future perspectives." Advances in therapy 33.1 (2016): 1-28.
[2] Bussi, Simona, et al. "Macrocyclic MR contrast agents: evaluation of multiple-organ gadolinium retention in healthy rats." Insights into imaging 11.1 (2020).
Figures