2484
Image Downsampling Expedited Adaptive Least-squares (IDEAL) fitting improves IVIM analysis in the human kidney
Julia Stabinska1,2,3, Helge Jörn Zöllner1,2, Hans-Jörg Wittsack3, and Alexandra Ljimani3
1F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 2The Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3Department of Diagnostic and Interventional Radiology, Heinrich Heine University Dusseldorf, Dusseldorf, Germany
1F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 2The Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3Department of Diagnostic and Interventional Radiology, Heinrich Heine University Dusseldorf, Dusseldorf, Germany
Synopsis
Recent studies have shown that a three-compartment model is preferable when analyzing renal DWI signal, as the conventional bi-exponential IVIM fitting does not differentiate between pure water diffusion and incoherent intra-tubular fluid motion. However, triexponential IVIM modelling in the kidney is challenging due to suboptimal SNR of diffusion-weighted images. In the present study, we applied Image Downsampling Expedited Adaptive Least-squared (IDEAL) fitting, which utilizes image downsampling to generate high SNR images to iteratively update the initial model parameters until the final image resolution is reached, and achieved more reliable estimates of the IVIM-related parameters compared with the conventional fitting methods.
Introduction
Conventional intravoxel incoherent motion (IVIM) analysis of diffusion-weighted signal utilizes a bi-exponential decay model to distinguish between capillary perfusion and tissue diffusion1. However, more recent studies have demonstrated that a three-compartment model might be preferable when performing DWI of the kidney2,3. It has been speculated that incoherent renal water motion is linked to three sources: (i) pure water diffusion in the renal tissue (ii) incoherent intra-tubular fluid motion, and (iii) incoherent blood motion, associated with the slow, intermediate and fast (pseudo-) diffusion components, respectively2.Triexponential IVIM modelling in the kidney remains challenging due to a limited b-value sampling and an inherently low SNR of diffusion-weighted images2. Its performance is also highly dependent on initial parameters and lower and upper parameter limits used for the constrained nonlinear data fitting. Fixing either one or two pseudo-diffusion coefficients is a common approach to improve fit stability, but it may introduce bias to the perfusion fraction2.
In this study, we applied an Image Downsampling Expedited Adaptive Least-squared (IDEAL) fitting algorithm that utilizes the high SNR of downsampled images for iterative tri-exponential fitting to improve the reliability of the of tri-exponential IVIM modelling of the human kidney.
Methods
Seven healthy volunteers (5 females and 2 males, mean age 30.1±5.4 years) were recruited for this study. DWI was performed on a 3T MRI system (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) using a respiratory-triggered single-shot DW-EPI sequence with the following parameters: 3 slices; TE/TR = 71/1900 ms; matrix: 176 x 176; voxel size: 2.1 x 2.1 x 5.0 mm3; averages: 3; diffusion directions: 3; 16 b-values distributed nonuniformly between 0 and 750 s/mm2.Image registration based on a normalized mutual information similarity measure was applied to correct for spatial misalignment of DW-images. Data analysis was performed using custom-written MATLAB routines. All 16 b-values were used to fit a triexponential model to the DW signal in each voxel for estimating the S0 image and IVIM parameters (Dslow, Dinterm, Dfast, finterm, and ffast). Three different fitting approaches were tested: (i) a conventional voxel-wise fit (Voxel), (ii) an optimized fit with fixed pseudodiffusion coefficients (Fixed D*)2, and (iii) an iterative IDEAL fit (IDEAL)5. The flow chart of the IDEAL fitting procedure is displayed in Figure 1. The DW images were downsampled to: 1x1, 2x2, 4x4, 8x8, 16x16, 32x32, 64x64, 96x96, 128x128, 152x152, till the original size of 176x176. The initial parameters of each voxel were determined by spatially interpolating the fitted parameters of the previous downsampled images. The lower and upper bounds were tightly constrained to 20% of the initial values for amplitude and 5% for the corresponding diffusion coefficients. Mean values and standard deviation of the cortical and medullary IVIM parameters and coefficient of variation (CV) within the ROIs in the fitted parameter maps were calculated.
Results
Compared with the voxel-wise and optimized fitting with fixed D*, the IDEAL method yields less noisy estimates of the f parameters, allowing better visualization of the complementary renal structures. A similar pattern was observed in all the imaged kidneys, in which the highest values of fslow were found in the medulla, finterm mostly corresponded to the cortex, renal columns and pelvis, and highest ffast was measured in the renal arteries and veins (Figure 2). The distribution of f and Dslow parameter values obtained in the cortex and medulla of the right kidney shows substantial reduction in variability for all ROIs with the IDEAL fitting compared with the voxel-wise fitting. While Fixed D* shows similarly low variance in fslow, finterm and Dslow estimates, it seems to slightly underestimate the fslow and Dslow parameters when compared with the remaining methods (Figure 3A and 3B). The coefficients of variance of the fitted cortical and medullary signal fractions and diffusion coefficient Dslow were substantially lower when IDEAL fitting was applied (Figure 4).Discussion
The IDEAL fitting method quantifies IVIM-related parameters based on starting values from tri-exponential fitting of iteratively downsampled images until quantitative IVIM parameters maps of original resolution are calculated. This approach provides accurate estimates of the IVIM parameters that are consistent with literature without signal averaging over all subjects or fixing any parameters2,3,4. This may be of particular importance when applying IVIM analysis to study renal pathologies in which substantial alterations in the physicochemical properties of blood and tissue are expected.The IDEAL algorithm uses tightly constrained lower and upper bounds after initial fitting, and is therefore less affected by low SNR than the conventional voxel-wise fitting method. As a result, the IVIM parameter maps obtained by the IDEAL algorithm show reduced coefficients of variation and higher contrast to noise ratio, which are essential for reliable detection and characterization of pathologies superimposed with normal renal tissue.
Conclusion
The IDEAL method improves the IVIM analysis using a tri-exponential model, allowing better visualization of the renal structures and more reliable estimates of the IVIM parameters than the conventional fitting methods.Acknowledgements
No acknowledgement found.References
- Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988 Aug;168(2):497-505.
- van Baalen S, Leemans A, Dik P, Lilien MR, Ten Haken B, Froeling M. Intravoxel incoherent motion modeling in the kidneys: comparison of mono-, bi-, and triexponential fit. J Magn Reson Imaging 2017;46:228-39.
- van der Bel R, Gurney-Champion OJ, Froeling M, Stroes ESG, Nederveen AJ, Krediet CTP. A tri-exponential model for intravoxel incoherent motion analysis of the human kidney: in silico and during pharmacological renal perfusion modulation. Eur J Radiol 2017;91:168-74.
- Stabinska J, Ljimani A, Zöllner HJ, Wilken E, Benkert T, Limberg J et al. Spectral diffusion analysis of kidney intravoxel incoherent motion MRI in healthy volunteers and patients with renal pathologies. Magn Reson Med. 2021 Jun;85(6):3085-3095.
- Zhou IY, Wang E, Cheung JS, Zhang X, Fulci G, Sun PZ. Quantitative chemical exchange saturation transfer (CEST) MRI of glioma using Image Downsampling Expedited Adaptive Least-squares (IDEAL) fitting. Sci Rep. 2017 Mar 7;7(1):84.
Figures
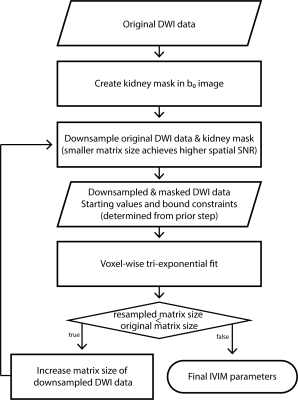
Figure 1: Flow chart of the IDEAL fitting method for IVIM data analysis.
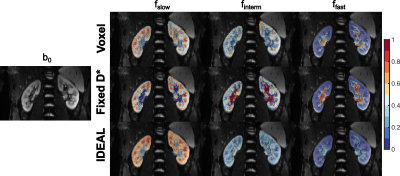
Figure 2: Signal fraction maps obtained using the conventional voxel-wise
fitting (Voxel), optimized fitting with fixed Dslow and Dfast
(Fixed D*), and iterative IDEAL fitting (IDEAL) in a representative healthy volunteer.
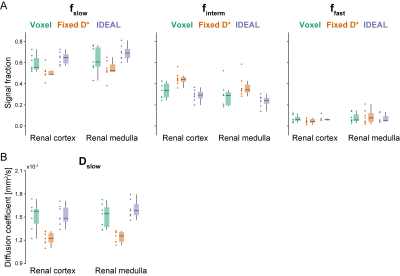
Figure 3:
Comparison of IVIM parameter estimates, (A) signal fractions (fslow,
finterm, ffast) and (B) diffusion coefficient (Dslow)
measured in the cortex and medulla of the right kidney in seven healthy
volunteers. Results are presented as individual datapoints and boxplots.
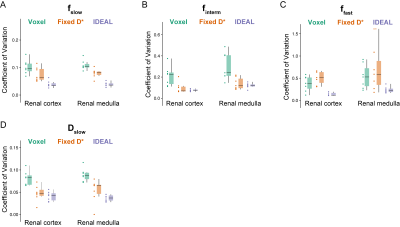
Figure 4:
Comparison of CVs of the fitted (A, B, C) signal fractions (fslow, finterm,
ffast) and (D) diffusion coefficient (Dslow) measured in
the cortex and medulla of the right kidney in seven healthy volunteers. Results
are presented as individual datapoints and boxplots.
DOI: https://doi.org/10.58530/2022/2484