2478
Brainwide functional networks associated with hippocampal subfields during memory task using fMRI with 1-mm isotropic resolution1Radiology, UNIV OF NORTH CAROLINA AT CHAPEL HILL, Chapel Hill, NC, United States, 2BRIC, UNIV OF NORTH CAROLINA AT CHAPEL HILL, Chapel Hill, NC, United States, 3Martinos center, Massachusetts General Hospital, Charlestown, MA, United States, 4Psychology, UNIV OF NORTH CAROLINA AT CHAPEL HILL, Chapel Hill, NC, United States
Synopsis
The interactions between the hippocampal subfields (HCSFs) and cerebral cortex during memory are unclear due to the inherently-low SNR, insufficient spatial resolution or limited spatial coverage. This study is the first fMRI study to examine how the HCSFs interact with the entire cortical surface during the memory encoding and retrieval process by addressing the above-mentioned issues successfully. Our results suggested that 1) the cortical-HCSF functional connectivity (FC) during encoding generally decreased compared to resting, 2) the cortical-HCSF FC increased compared to encoding during retrieval; 3) stronger FC between posterior CA1 and dorsal attention network are correlated with better behavioural performance.
Introduction
Hippocampus (HC) plays a major role in memory, and different hippocampal subfields (HCSFs) support memory encoding and retrieval processes differently. A number of fMRI studies demonstrated the event-related BOLD actions either at the hippocampus1 or on the cortical surface. However, the interactions between the HCSFs and cerebral cortex during memory are unclear mainly due to the technical challenges. Studying the brain-wide functional connectivity (FC) of HCSFs requires ultrahigh-resolution functional imaging of which the signal-to-noise ratio (SNR) is inherently low. Meanwhile, images encompassing the entire brain and acquisition within a few seconds are needed. To address the SNR and coverage issues, we employed 1) the MPPCA method which has been shown to be effective in thermal noise removal2 and 2) the simultaneous multislice (SMS) method with the phase-encoding (PE) axis along superior-inferior direction to achieve TE of 23 ms, 1-mm isotropic resolution, whole-brain coverage, and repetition time (TR) of 2s. To characterize cortical-HCSF interactions during various cognitive processes, fMRI data were acquired at rest and during a memory task.Method
We recruited 19 healthy adults aged 18 to 30 years old (20.8±3.6 years; 8 males). MR images were acquired using a Siemens 7T Magnetom scanner. To accommodate the short echo time (TE) at 7T, the PE is oriented along the superior-inferior direction with a FOV saturation band placed axially immediately below the image FOV. The spatial resolution=1 mm isotropic, field-of-view (FOV)=120 x 152 x 175 mm3, the total number of coronal slices=175, TE=23 ms, TR=2s, SMS factor=7, in-plane acceleration factor=2. Two 6-minute resting-state scans, followed by 6 pairs of encoding and retrieval runs. The MPPCA method was implemented using an in-house MATLAB code and applied before the parallel-imaging reconstruction. The labels of HPSFs were obtained using Freesurfer 6.0. The presubiculum, subiculum, CA1, CA3, CA4/DG are subdivided into head and body portions in consideration of longitudinal functional specialization3-4.At encoding, participants viewed 42 name-object pairs for 3500ms each. During the retrieval runs that immediately followed the encoding run, participants saw a previously studied name (cue) for 1000ms followed by the object (probe) appear beneath the name for 2000 ms and were told to respond via button press whether the image was the same as (target, “same”), very similar to but not exactly the same as (lure, “similar”), or very different from (novel, “different”) the image previously paired with the name.
The seed-based functional connectivity was calculated using Pearson correlation coefficient. After transforming the correlation coefficient to Fisher’s z values, the statistical significance of the group-averaged FC was calculated using the Freesurfer command ‘mri_glmfit’. Correcting for multiple comparisons was performed by the Monte Carlo Simulation in FreeSurfer. The behavioural correlates were measured between the Fisher’s z values and the accuracy of in-scanner tasks. The statistical significance of behavioural correlates was calculated using ‘mri_glmfit’ and Monte Carlo Simulation for multiple-comparison corrections.
Results
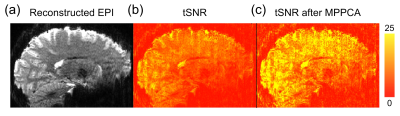
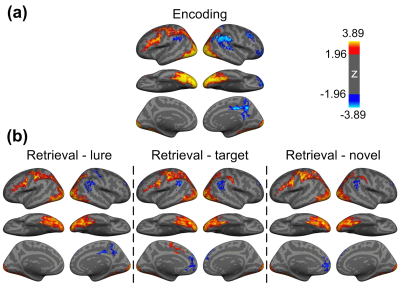
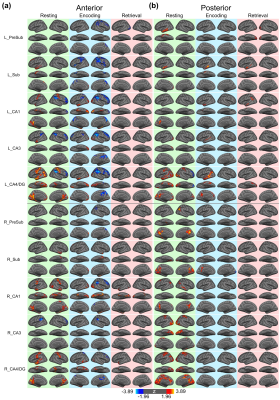
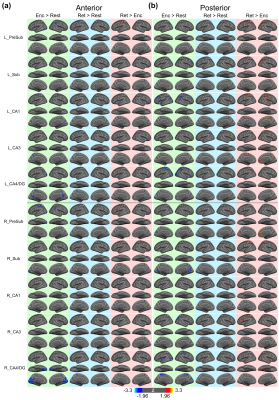
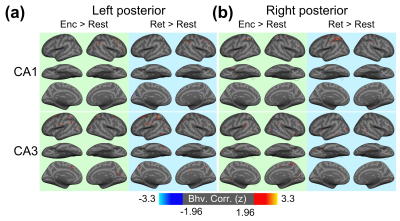
Figure 1 shows the reconstructed EPI and the temporal SNR (tSNR) before and after the MPPCA denoising. Figure 2 shows the whole-brain event-related responses associated with different task conditions, demonstrating BOLD activations at visual area, superior parietal lobule, frontal eye field (FEF), dorsal lateral prefrontal cortex (DLPFC), and fusiform gyrus. Figure 3 demonstrates the whole-brain FC maps associated with all the HCSFs (corrected p < 0.05). To understand how the hippocampal subfields interact with the entire cortical surface during various cognitive processes, we took the absolute values of the FCs and contrasted between rest, encoding, and retrieval, i.e. Δ|FC|. The statistical maps of Δ|FC| are shown in Figure 4. Prominent behavioral correlates with Δ|FC| are shown in Figure 5. Our results suggested that 1) the cortical-HCSF FC strength during encoding generally decreased compared to resting, particularly within the visual, sensorimotor, frontoparietal networks; 2) the cortical-posterior HCSF FC strength during retrieval generally increased compared to encoding, particularly within the sensorimotor, frontoparietal, and default-mode networks; 3) stronger FC strength between posterior CA1 or CA3 and selective regions within dorsal attention or frontoparietal networks are correlated with better behavioural performance. Specifically, the selective regions within dorsal attention network include superior/inferior left parietal lobe, left frontal eye field (FEF) which showed event-related BOLD responses during memory task.Discussions
This study is, to the best of our knowledge, the first to examine how the HCSFs interact with the entire cortical surface during the memory encoding and retrieval process. The changes in FC strength during encoding and retrieval were observed. Based on the synchronization–desynchronization framework5-7, the neocortical alpha/beta (8 to 20 Hz) power will decrease and the hippocampal gamma power will increase during episodic memory process. The observed change of FC strength may reflect the BOLD synchronization upon the alterations of cortical and hippocampal neural oscillatory power5-7. The significant behavioural correlation with the FC between CA1/CA3 and dorsal attention networks is in line with the intracranial electroencephalogram studies which reported that the oscillatory power at CA1 and CA3 predict the success of memory retrieval6. In conlusion, this study provides the insights about the brainwide cortical-HPSF functional networks during memory process and the findings on healthy young adults could serve as an important reference for the healthy and pathological aging.Acknowledgements
This work was supported in part by NIH grants, R21AG060324 and U01MH110274.
References
Figures