2476
Static and dynamic connectivity are differentially affected by head motion: a healthy control and Parkinson’s disease study
Francesca Saviola1, Stefano Tambalo1, Donna Gift Cabalo1, Lisa Novello1, Enrica Pierotti1, Alessandra Dodich1, Costanza Papagno1, Luca Turella1, Dimitri Van De Ville2,3, and Jorge Jovicich1
1University of Trento, Center for Mind/Brain Sciences, Rovereto, Italy, 2Institute of Bioengineering, Center for Neuroprosthetics, Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 3Department of Radiology and Medical Informatics, University of Geneva (UNIGE), Geneva, Switzerland
1University of Trento, Center for Mind/Brain Sciences, Rovereto, Italy, 2Institute of Bioengineering, Center for Neuroprosthetics, Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 3Department of Radiology and Medical Informatics, University of Geneva (UNIGE), Geneva, Switzerland
Synopsis
An open discussion in functional connectivity (FC) studies is the mitigation of motion-related artifacts. Data-driven denoising such as Independent Component Analysis (ICA) could help in improving the reproducibility of results, however, the definition of pipelines to deal with mild to high motion cases is still controversial. We estimate the effect of different workflows optimized for best-controlling head motion both in healthy and Parkinson’s Disease cohorts. Regardless of baseline head motion level, ICA-based control of motion confounds affects functional connectivity metrics, with non-negligible impact on static connectivity and most severe effects on temporal and spatial features of dynamic functional connectivity measures.
Introduction
Head motion is one of the major confounds in cross-sectional fMRI studies[1-3], particularly in presence of peculiar symptomatology as in the case of Parkinson’s disease (PD). Previous studies show that Independent Component Analysis (ICA) denoising improves the reproducibility of clinical findings[4] by detecting sources of non-neural signals. However, there is still no consensus about which pre-processing pipeline should be applied in high motion cases such as PD patients, taking into account the well-known effects of this choice on the consequent results[5]. In this study we aim to investigate the effect of different pre-processing pipelines for best-controlling head motion confounds both in healthy controls (HC) and PD patient cohorts for the estimation of brain functional connectivity, both static and dynamic.Materials and Methods
A total of 20 HC (10 females; age 24±3 years) and 14 PD patients (7 females; age 64±8 years) participated in this study. Data were acquired with 3T Siemens Prisma MRI scanner equipped with a 64 channels receive-only head coil. Structural T1-weighted multi-echo MPRAGE for the PD cohort (TR=2.5s, TEs=[1.69, 3.55, 5.41, 7,27 ms], 1mm-isotropic voxels), standard MPRAGE for the HC group (TR/TE=2.31s/3.48ms, 1mm-isotropic voxels) and resting-state functional MRI (rs-fMRI, TR=1s, TE=28 ms, 3mm-isotropic voxels) images were used to test the effects of the preprocessing pipeline of static (sFC) and temporal-varying functional connectivity (dFC) estimations. Both MRI datasets were pre-processed using three different workflows. Common pre-processing steps included: (1) slice timing and head motion correction; (2) co-registration of the T1-weighted image to the rs-fMRI time-series; (3) T1-weighted image tissue segmentation; (4) rs-fMRI temporal detrending and median filtering; (5) regression from the rs-fMRI time-series of the 6 head motion parameters, white matter and cerebro-spinal fluid signals (standard motion correction, mc); (7) normalization to standard MNI template space; and (8) spatial smoothing 6 mm FWHM Gaussian kernel size. Together with standard pre-processing steps, the three pipelines differed in the motion correction approach: (i) mc; (ii) motion outliers detection and deweighting based on DVARS estimations[6] (DVARS); (iii) noise removal by means of ICA-AROMA[7] (AROMA). sFC analysis was performed using Group ICA in MELODIC, by temporally concatenating different pre-processing types in two different runs, respectively mc-DVARS and mc-AROMA, to then perform a paired t-test in each group separately.dFC analysis was computed through innovation-driven co-activation patterns (iCAPs) (https://c4science.ch/source/iCAPs/) which allows the estimation of: (i) recurrent brain states maps (iCAPs, k=11); (ii) total duration percentage of each functional network (iCAP). Durations between pipelines were compared using a paired t-test for the two different runs described above for both groups (HC and PD separately). To gain insights about the effect of DVARS and AROMA with respect to mc, a two-sample unpaired t-test was performed for both groups respectively on: (i) a linear combination of the regressed outlier volumes for DVARS; (ii) the algebraic sum of the Independent Components regressed out from the time-series by AROMA.Results
Head motion metrics statistically differed in the two groups (mean framewise displacement (mm): HC (3.4土1.2), PD (9.2土3.8), p<0.001). Regarding sFC, in both HC and PD,mc and DVARS do not statistically differ in all retrieved resting-state networks (RSNs). Instead, relative to mc, AROMA shows significant voxelwise differences for all RSNs both in regions expected to be motion-sensitive (Fig. 1A) and not (Fig. 1B-C). Regarding dFC in the HC group, from a spatial point of view, the three motion correction pipelines retrieved iCAPs maps of well-known RSNs (Fig. 2), although with low accuracy for anterior Salience network (aSN) in DVARS and Visuospatial (VSN) network in mc. From a temporal perspective, HC show differences in engagement of a given state throughout the timecourse for the different runs (Fig. 3): (i) for Auditory, Visual, aSN and dorsal default mode networks total duration is increased in mc compared to DVARS and compared to AROMA (pFDR<0.05), which also show a disruption in the Insula network, (ii) on the other hand, the VSN, which was poorly retrieved in mc, had a reduced total duration compared to DVARS and AROMA (pFDR<0.05). In the PD cohort, dFC is severely affected by the motion correction pipeline (Fig. 4), resulting in a disruption of both the spatial distribution and the temporal features of brain networks when choosing DVARS (two iCAPs detected) and AROMA (one iCAP detected) compared to mc (five iCAPs detected). Voxelwise statistical maps of the noise components removed by AROMA and DVARS (Fig. 5) show a clear increase in effect of AROMA denoising in PD compared to HC (pFWE<0.05).Discussion and Conclusions
Within two groups having different head motion levels (HC and PD), we evaluated how standard, DVARS and AROMA head motion denoising methods affect sFC and dFC. We found that static FC metrics are mostly robust across motion denoising strategies in both groups[8,9]. Dynamic FC metrics were very sensitive to denoising methods, especially in the Parkinson’s group. In particular, both AROMA and DVARS denoising severely disrupted the temporal properties of dynamic networks in the PD. Further work is needed to better understand: (i) how to retain useful information from a BOLD time-series in high motion populations, (ii) and to disentangle subject-specific dynamic head motion signatures potentially retaining relevant neural information[10,11].Acknowledgements
ISMRM Exchange Award 2021-2022 “Investigating in-vivo human brain dynamic connectivity with fast fMRI”, Dipartimento di Eccellenza project 2018-2022 (Italian Ministry of Education, University and Research).References
- Siegel, J. S., Power, J. D., Dubis, J. W., Vogel, A. C., Church, J. A., Schlaggar, B. L., & Petersen, S. E. (2014). Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high‐motion data points. Human brain mapping, 35(5), 1981-1996.
- Power, J. D., Plitt, M., Laumann, T. O., & Martin, A. (2017). Sources and implications of whole-brain fMRI signals in humans. Neuroimage, 146, 609-625.
- Murphy, K., Birn, R. M., & Bandettini, P. A. (2013). Resting-state fMRI confounds and cleanup. Neuroimage, 80, 349-359.
- Carone, D., et al. "Impact of automated ICA-based denoising of fMRI data in acute stroke patients." NeuroImage: Clinical 16 (2017): 23-31.
- Griffanti, L., Rolinski, M., Szewczyk-Krolikowski, K., Menke, R. A., Filippini, N., Zamboni, G., ... & Mackay, C. E. (2016). Challenges in the reproducibility of clinical studies with resting state fMRI: An example in early Parkinson's disease. Neuroimage, 124, 704-713.
- Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L., & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage, 59(3), 2142-2154.
- Pruim, R. H., Mennes, M., van Rooij, D., Llera, A., Buitelaar, J. K., & Beckmann, C. F. (2015). ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage, 112, 267-277.
- Raval, V., Nguyen, K. P., Pinho, M., Dewey, R. B., Trivedi, M., & Montillo, A. A. (2021). Pitfalls and recommended strategies and metrics for suppressing motion artifacts in functional MRI. bioRxiv.
- Mahadevan, A. S., Tooley, U. A., Bertolero, M. A., Mackey, A. P., & Bassett, D. S. (2021). Evaluating the sensitivity of functional connectivity measures to motion artifact in resting-state fMRI data. NeuroImage, 241, 118408.
- Xifra-Porxas, A., Kassinopoulos, M., & Mitsis, G. D. (2021). Physiological and motion signatures in static and time-varying functional connectivity and their subject identifiability. Elife, 10, e62324.
- Bolton, T. A., Kebets, V., Glerean, E., Zöller, D., Li, J., Yeo, B. T., ... & Van De Ville, D. (2020). Agito ergo sum: Correlates of spatio-temporal motion characteristics during fMRI. Neuroimage, 209, 116433.
Figures
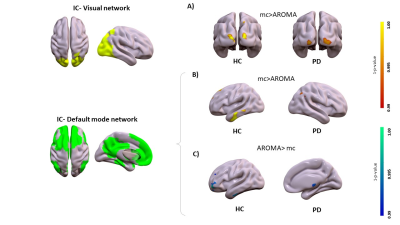
Motion correction effects on static networks in healthy controls and Parkinson’s disease. Spatial patterns of the significant voxelwise changes in static functional connectivity while comparing mc to AROMA. Panel A, significant changes in the visual network for mc>AROMA in head motion-sensitive areas (p<0.001Bonferroni). Panel B and C, significant changes in the default mode network respectively from mc>AROMA and AROMA>mc (p<0.001Bonferroni).
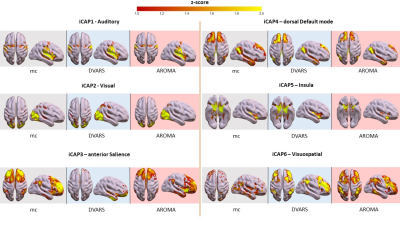
Motion correction effects on dynamic networks in healthy controls. Spatial patterns of the 11 innovation-driven coactivation patterns (iCAPs) in the healthy control group were retrieved from all subjects in all sessions (grey: mc, blue: DVARS; red: AROMA).
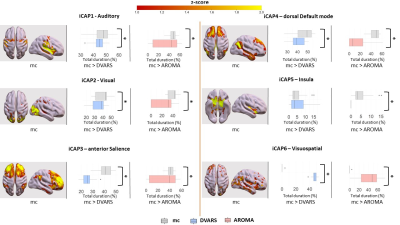
Motion correction effects on duration of dynamic networks in healthy controls. For each iCAPs (spatial maps from mc), horizontal boxplots show the innovation frames per session in the healthy control group as a percentage of the total scanning time, highlighting the differences between: (i) mc and DVARS runs (*=p<0.05FDR) in the second column, (ii) mc and AROMA runs (*=p<0.05FDR) in the third column.
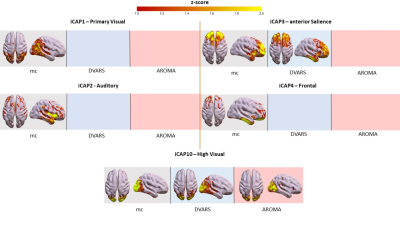
Motion correction effects on duration of dynamic networks in Parkinson’s disease. Spatial patterns of the 11 innovation-driven coactivation patterns (iCAPs) in PD group retrieved from all subjects in different sessions (grey: mc, blue: DVARS; red: AROMA).
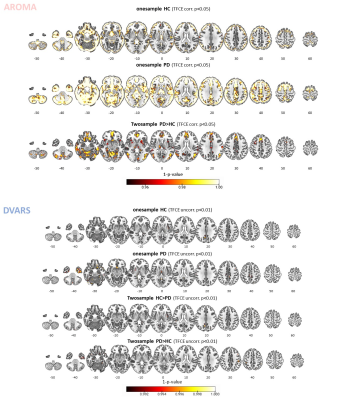
Examination of removed noise components. Maps of connectivity components removed by the AROMA (upper part) and DVARS (bottom part) pipelines within the healthy control (HC), Parkinson’s disease (PD) groups, and their relative comparison. The results show how AROMA removes a larger amount of connectivity considered noise, especially in the PD group, which in parts resembles resting-state networks, such as the default mode network. Instead, DVARS removes much less and similar levels across the two groups.
DOI: https://doi.org/10.58530/2022/2476