2475
Structural and resting-state functional connectivity changes due to chronic noise exposure1Department of NMR and MRI Facility, All India Institute of Medical Sciences, New Delhi, India
Synopsis
The effects of cumulative lifetime noise exposure on ascending auditory pathway function in audio metrically normal individuals is explored by sustained and transient fMRI responses and related auditory brainstem response (ABR). The experiment included continuous (scanner noise) and deviant noise (additional pink noise) levels that affected mental workload and auditory/visual attention. Our results exhibited reduced attention and mental workload in central auditory regions in people exposed to higher noise (>85dBA) in comparison with those exposed to lesser noise (<85dBA).
Introduction
Chronic noise exposure induces cognitive impairment and oxidative stress in the brain1. Impact of noise on cognitive function has been the subject of much debate, considering road rage or aggressive behaviour in certain individuals exposed to noise. Lower sound-level tolerance resulted in a higher sustained fMRI response in the inferior colliculus (IC) and medial geniculate body (MGB) in response to continuous broadband noise, ascribed to central gain augmentation2. It is well established that subcortical regions (such as the IC) respond to continuous sounds with a sustained fMRI response3, whereas the main auditory cortex response is yet to be explored. By inducing continuous (scanner noise) and deviant noise (additional pink noise) we studied their effects on mental workload and auditory/visual attention using fMRI and auditory brainstem response (ABR) in healthy population.Method
Study was conducted on 52 healthy subjects (13 Females & 39 Males, mean age 29.13±7.43 years), categorized into three groups i.e., Low (<60dB), Medium (65-85dB) and High (>85dB) noise exposure groups (all had exposure up to 10hrs/day).Data acquisition: MR imaging (3D T1) and post task rsfMRI studies were carried out on a 3 T MR scanner (Ingenia 3.0T TX, M/s. Philips Medical Systems) using a 32 channel head coil. The BOLD imaging was carried out using single shot echo planar imaging sequence with 35 slices of 4.0 mm thickness, flip angle = 90°, transverse orientation, fold-over direction: AP, field of view = 230 mm with TR/TE = 2000/30 ms, number of dynamics = 205.
Data analysis: Structural (T1) data was processed with the CAT12 toolbox within SPM12 (version CAT12.6) to extract surface based cortical thickness (CT) and gyrification index (GI) estimates. Resting state functional connectivity was evaluated using the CONN toolbox (Ver. 19) with ROI-to-ROI analysis using the General Linear Model and correlation4. Results are presented with Holm-Bonferroni correction and at p<0.05, FDR corrected.
Result
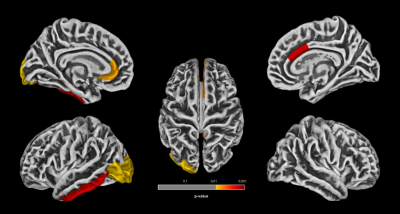
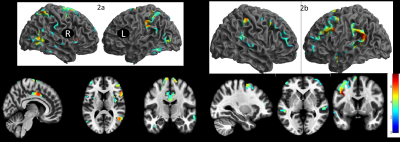
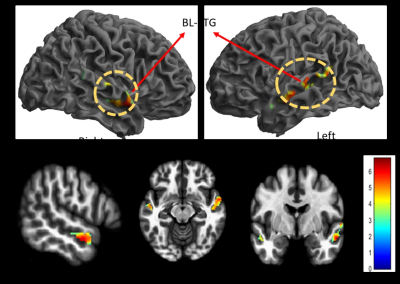
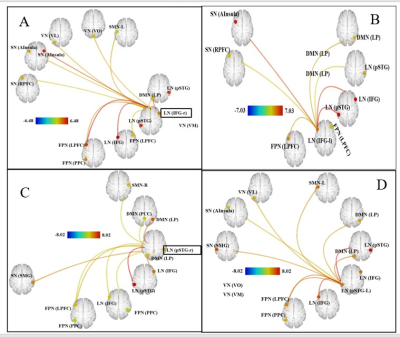
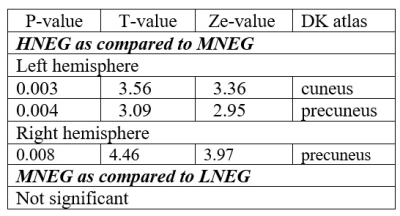
Gyrification indices and total intracranial volume revealed significant differences between high noise exposed group (HNEG) and medium noise exposed group (MNEG), but there was no difference between MNEG and low noise exposed groups (LNEG) (Figure 1, Table 1). A widespread increase in gray matter volume (GMV) was observed in various areas of brain in HNEG as compared to MNEG and LNEG (Figure 2). Increased GMV in frontal, temporal, parietal and occipital regions was evident in high noise exposed group. Significant differences in MNEG as compared to LNEG was observed in bilateral speech and language areas (Figure 3). The ROI analysis in HNEG exhibited increased functional connectivity between the right lateral pre-frontal cortex (LPFC) of the fronto-parietal (FP) network and bilateral supramarginal gyrus nodes of the substantia nigra (SN), and decreased FC between the medial pre-frontal cortex (MPFC) and the left lateral parietal (LP) node of the default mode network (DMN) (Figure 4). Bivariate correlations between right LPFC and bilateral attentional network were observed in HNEG with respect to LNEG. When comparing MNEG with LNEG, both revealed increased connectivity of the SN left anterior insula (AI) with all four FP network nodes, with a shared connectivity of right LPFC and PPC nodes with the left anterior insular region.Discussion
Noise has an effect on cognitive function and brain signals3. When people are exposed to different degrees of noise, their mental effort, visual/auditory attention, and the relative power of the frequency bands all follow a similar pattern3. We explored the anatomical and functional changes in the brain of people who have had high, medium or low levels of noise exposure. An ROI-to-ROI analysis of the noise-exposed network based on post-fMRI task brain mapping demonstrated intact connectivity throughout the brain and no atrophy-related changes in the three groups. On intergroup comparison, the results revealed a discriminating connectivity in between the three groups, which included the hearing, speech, and attention networks. When compared to the LNEG, high noise exposure resulted in increased connectivity and gray matter areas in the auditory, language, attention networks, salience networks, and frontoparietal network (cognitive control). In comparison to the LNEG, the default mode network showed strong intrinsic connectivity in the MNEG. The bilateral supplementary motor area and left insula were similar to all three groups, according to a multimodal assessment of the spatial overlap of the structural and two functional outcomes. Auditory perception and discrimination accuracy were found to be significantly different in the HNEG compared to the LNEG in neural and behavioral investigations, implying that the high exposed group had supra-normal capacity to execute in higher order auditory attention tasks5. The medial geniculate body's dorsal area and the auditory cortex's association area are critical for maintaining and directing auditory attention. In a complex multisource auditory scene, the process of selectively directing attention to a single auditory stream may actually affect our perceptual structure of the scene's features. Overall, differentiating foreground from background and extracting signal from noise is likely to be a multi-stage process including bottom-up gestalt grouping primitives, auditory memory, attention, and other forms of top-down control6.Conclusion
The results showed individuals exposed to high noise (at a level of <85 dBA) exhibited reduced mental workload and visual/auditory attention, revealing the effects of noise exposure on cognitive performance.Acknowledgements
The funding from Life Sciences Research Board (LSRB), Ministry of Defence, Government of India is duly acknowledged (vide grant No. LSRB-295/PEE&BS/2017)References
1. Jafari, M. J., Khosrowabadi, R., Khodakarim, S., & Mohammadian, F. (2019). The Effect of Noise Exposure on Cognitive Performance and Brain Activity Patterns. Open access Macedonian journal of medical sciences, 7, 2924–2931.
2. Gu, J. W., Halpin, C. F., Nam, E. C., Levine, R. A., & Melcher, J. R. (2010). Tinnitus, diminished sound-level tolerance, and elevated auditory activity in humans with clinically normal hearing sensitivity. Journal of neurophysiology, 104, 3361–3370.
3. Dewey, R. S., Francis, S. T., Guest, H., Prendergast, G., Millman, R. E., Plack, C. J., & Hall, D. A. (2020). The association between subcortical and cortical fMRI and lifetime noise exposure in listeners with normal hearing thresholds. NeuroImage, 204, 116239.
4. Whitfield-Gabrieli S, Nieto-Castanon A. (2012) Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect., 2:125-41.
5. Dommes, E., Bauknecht, H. C., Scholz, G., Rothemund, Y., Hensel, J., & Klingebiel, R. (2009). Auditory cortex stimulation by low-frequency tones-An fMRI study. Brain Research, 1304, 129-137.
6. Hommel, B., Fischer, R., Colzato, L. S., Van Den Wildenberg, W. P., & Cellini, C. (2012). The effect of fMRI (noise) on cognitive control. Journal of Experimental Psychology: Human Perception and Performance, 38, 290.
Figures