2471
Brain functional fingerprinting predicts individual differences in cognition: An AI-based approach1Department of Diagnostic Imaging, Akershus University Hospital, Lørenskog, Norway, 2Department of Electrical Engineering and Computer Science, University of Stavanger, Stavanger, Norway, 3Wallenberg Center for Molecular Medicine (WCMM), Umeå University, Umeå, Sweden, 4Department of Integrative Medical Biology, Umeå University, Umeå, Sweden, 5Aging Research Center, Karolinska Institute and Stockholm University, Solna, Sweden
Synopsis
Machine learning approaches provide convenient autonomous object classification in medical imaging domains. This study examines the utility of convolutional neural networks in predicting individual differences in cognition from the resting-state functional connectome. We observed significant contributions from the subcortical areas (including hippocampus) and their interactions with the cortical default mode network to the training progress. Our results demonstrate that an AI-based model can predict an individual's EM scores.
INTRODUCTION
Functional MRI enables the identification of several resting-state networks, constituting the brain's functional architecture. This functional architecture alters in different conditions such as aging and Alzheimer's disease, suggesting that functional connectivity measures may serve as sensitive biomarkers. Previous studies suggested that connectome-based predictive modeling could predict cognition 1, but the accuracy for prediction was relatively moderate and largely dependent on several technical choices, such as feature selections. This study aims to develop artificial intelligence (AI) platform to predict individual behavior, such as episodic memory (EM) score, from individual functional connectivity profiles. The secondary aim is to identify the features of the deep convolutional neural network model that contributed to predictions of EM.METHODS
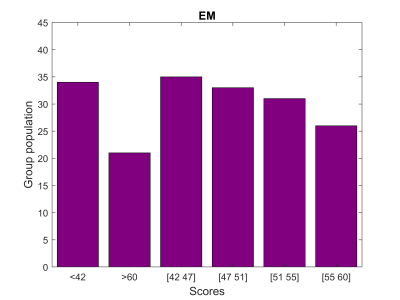
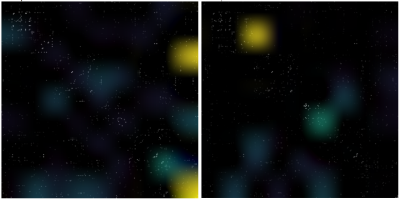
We present resting-state functional connectivity acquired from 180 healthy subjects (age: 20-78 y old, 50% female) of the DopamiNe, Age, connectoMe, and Cognition (DyNAMiC) project, a multimodal, prospective 5-year longitudinal study spanning the adult human lifespan (eBusiness Bootstrap Template (alirezasalamilab.com)). This study was approved by the Regional Ethical board and the local Radiation Safety Committee in Umeå, Sweden. Functional data were obtained during the rest-state conditions (~12 min). The details of the pre-processing pipeline are described elsewhere 2, resulting in functional architecture maps of 273 x 273 connectivity nodes (Fig. 1). We removed the covariates of no interest (e.g., age) by predefined thresholds on the connectome features and partial-cross-correlation method 2. Following standard augmentation, we generated 180,000 functional connectivity maps categorized in six identical groups (Fig. 2). We modified internal loops inside the DenseNet-121 3 model (reducing dense block loop in one-third in size), resulting in a less dense model named semi-DenseNet. We trained the semi-DenseNet model with three-fold cross-validation by randomly shuffling data for training, validation, and testing patches. Three comparable groups with dataset distribution of 55%, 30%, and 15% for training, validation, and testing, respectively, were prepared for training. Each fold of cross-validation and testing was a new training phase based on an identical combination of the three groups. We held out the testing fraction of the dataset from the in-training step. Thus, the accuracy of models was calculated using the mean value of the network performance on only the testing dataset. The model used the Adam optimizer with a learning rate of 6e – 4 with a decay rate of 0.9. The batch size and number of epochs were 250 and 100, respectively. All experiments were computed on a computer equipped with Intel Corei7 CPU, Nvidia GeForce RTX 2080Ti GPU, and 32 GB of memory. We used the Grad-CAM algorithm 4 to visualize the model reasoning on aimed classification. The Grad-CAM generates visual explanations of post-processed image space gradients to heatmaps. The overlap of the generated heatmaps over the functional connectivity maps was used to explore connectomic features, which significantly contributed to the model predictions.RESULTS
The semi-DenseNet model predicted the EM score range based on connectivity map features with a mean cross-validated accuracy of >96% on the testing dataset. Grad-CAM-derived saliency heatmaps demonstrated significant contributions from subcortical nodes, particularly along the hippocampus axis (Fig. 3). Furthermore, the between-network connectivity of the default mode to the subcortical network significantly contributed to the predictive model (Fig. 3).DISCUSSION
Our AI-predict model showed that regions within the subcortical areas, including the hippocampus, and their interactions with the cortical default mode network, contributed to the prediction of individual differences in EM. This is in line with several previous studies showing that interactions between the default mode network regions have implications for memory function 5,6. Taken together, an AI-based model tool for predicting various behavioral measures may be of clinical relevance and interest for neuro-scientist to discover brain functionalities.Acknowledgements
This work was funded by the Swedish Research Council (grant number 2016-01936 to A.S.), Knut and Alice Wallenberg Foundation (Wallenberg Fellow grant to A.S.), bank of Sweden (RJ, grant number P20-0515 to A.S.), StratNeuro grant at Karolinska Institutet (A.S.), and Helse Sør-Øst RHF – Southern Eastern Norway Regional Health Authority (HSØ, grant number 2018047 to M.E.).References
1. Finn ES, Shen X, Scheinost D, et al. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci. 2015;18(11):1664-1671.
2. Pedersen R, Geerligs L, Andersson M, et al. When functional blurring becomes deleterious: Reduced system segregation is associated with less white matter integrity and cognitive decline in aging. Neuroimage. 2021;242:118449.
3. Huang G, Liu Z, Maaten Lvd, Weinberger KQ. Densely Connected Convolutional Networks. arXiv. 2016.
4. R. SR, Michael C, Abhishek D, Ramakrishna V, Devi P, Dhruv B. Grad-CAM: Visual Explanations from Deep Networks via Gradient-Based Localization. International Journal of Computer Vision. 2019;128.
5. Kaboodvand N, Backman L, Nyberg L, Salami A. The retrosplenial cortex: A memory gateway between the cortical default mode network and the medial temporal lobe. Hum Brain Mapp. 2018;39(5):2020-2034.
6. Salami A, Pudas S, Nyberg L. Elevated hippocampal resting-state connectivity underlies deficient neurocognitive function in aging. Proc Natl Acad Sci U S A. 2014;111(49):17654-17659.
Figures