2469
Neural correlates of fluid intelligence identified by empirical neural network-based brain states1Institute of Diagnostic and Interventional Radiology and Neuroradiology, University Hospital Essen, Germany, Essen, Germany, 2Department of Neurology, University Hospital Essen, Germany, Essen, Germany
Synopsis
We propose a novel method which considers the functional connectome as an already-trained, empirical continuous Hopfield Network, to extract brain states from a population connectome to analyze the dynamics of the so-called attractor states on the subject level. We apply our method to the Human Connectome Project dataset, and we could show, that the mean activation of the participants during different states is a significant predictor of fluid intelligence.
Introduction
The recent concept of activity-flow (Cole et al.) establishes a link between brain activity and functional connectivity by modelling regional activity as the sum of other regions’ activity, weighted by the functional connectivity between the regions1. We propose to use the activity-flow principle in an iterative way, which is equivalent to considering the resting-state functional connectome as an already trained continuous Hopfield Network2. Hopfield Networks are a type of recurrent feedback neural network that can serve as an ‘associative memory network’, originally applied for image reconstruction or classification. Conventionally, they are trained with known input patterns and when presented with a fragment of a stored pattern, the network can recall the originally stored pattern by relaxing into so-called attractor states. Here, instead of an explicit training procedure, we initialize the weights of a continuous Hopfield Network with functional connectivity values and hypothesize that the attractor states of this networks extract rich information about the connectome’s architecture and dynamic capabilities. To test out hypothesis, we (i) obtain population-level connectivity estimates from the Human Connectome Project and generate the attractor states for each time-frame of each participant’s resting-state fMRI data, (ii) consider these attractor states as “brain states”, (iii) test the association of the average, participant-level activity of these brain states with fluid intelligence.Methods
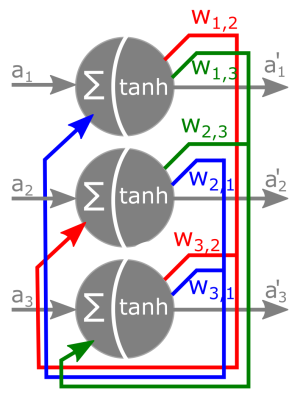
Hopfield Networks are recurrent artificial neural networks with a single fully connected layer, an input, and an output layer (Figure 1). The weight matrix W holds the connectivity values between each of the nodes, which contains information about the stored patterns within the network. The sigmoid activation function allows the network to produce continuous states, in contrast to the classical, binary Hopfield network. When presented with a pattern a, the network updates the output pattern a’ until a convergence criterium is met. The parameter β is a tunable hyperparameter often referred to as the temperature of the network, which controls the number of states that emerge from the trained network. We developed a method, that utilizes the functional connectome of resting-state fMRI data as the network weights of a continuous, empirical Hopfield Network. We initialize the network’s weights with a partial correlation-based connectome and consider it to be already trained to store unknown states. These unknown states can be uncovered by presenting the network with arbitrary activation patterns and running the relaxation procedure. The attractor states emerge in pairs, where one state is the inverse of the other. To test our hypothesis, we obtained functional connectivity data from the Human Connectome Project (HCP)3. Preprocessed resting state fMRI timeseries (ICA-based parcellation with 50 components4) was obtained as published with the HCP1200 release (N=812). The population-mean functional connectivity matrix was normalized (zero mean, unit variance) and used to initialize a Continuous Hopfield Network, with beta=0.08 (optimized in order to produce 4 attractor states). Each time-frame of the individual participants’ rsfMRI timeseries was considered as an “input” for this Hopfield Network and the corresponding attractor state was obtained. Time-frames belonging to the same attractor state were averaged to obtain the state-specific mean activation pattern for each participant. For each region of each state, state-specific mean activation was contrasted to the fluid intelligence score of the participants, as measured with Penn Progressive Matrices5. P-values were obtained with permutation testing and corrected for multiple comparisons across regions and brain states.Results
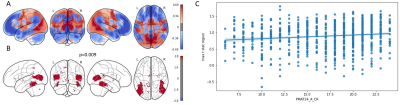
Comparing the null distribution of the permuted t-statistics to the unpermuted case, one pair of opposing attractor states showed statistical significance of p = 0.009 (corrected for FWER) and p < 0.001 respectively (see Figure 2). Given the Penn Progressive matrices' visual geometric design and the known role of the lateral occipital cortex's in object recognition6, the strong lateral occipital involvement in the associated attractor state can be considered as a sign of neurobiological relevance. The contribution of the precuneus, the angular gyrus and prefrontal regions are, furthermore, in line with the previously reported association of the fronto-parietal and executive control network activity to fluid intelligence7.Discussion
The results reinforce previous work reporting an association between resting state brain activity/connectivity and fluid intelligence 6, 7. Specifically, our results suggest that in epochs during which the visual and salience-related regions tend to co-activate (or de-activate simultaneously), the activation of the occipitoparietal association cortices/regions tends to be higher (lower) in participants with higher fluid intelligence.Conclusion
Our preliminary findings suggest that considering the functional connectome as an empirical Hopfield Network can be considered as a novel tool for identifying neurobiologically valid brain states. More elaborated analysis of attractor states holds promise for improving our understanding of the link between the complex network structure and the dynamical properties of the functional connectome.Acknowledgements
This research was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Projektnummer 316803389 – SFB 1280 and TRR 289 Treatment Expectation - Projektnummer 422744262.
Data were provided [in part] by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University.
References
1. Activity flow over resting-state networks shapes cognitive task activations. Michael W Cole, Takuya Ito, Danielle S Bassett, & Douglas H Schultz. 2016. Nature Neuroscience.2. Neural networks and physical systems with emergent collective computational abilities. Hopfield, J J. 1982. Proc Natl Acad Sci USA.
3. The WU-Minn Human Connectome Project: An overview. David C. Van Essen, Stephen M. Smith, Deanna M. Barch, Timothy E.J. Behrens, Essa Yacoub, Kamil Ugurbil, for the WU-Minn HCP Consortium. Neuroimage, 2013.
4. The minimal preprocessing pipelines for the Human Connectome Project. Matthew F Glasser, Stamatios N Sotiropoulos, J Anthony Wilson, Timothy S Coalson, Bruce Fischl, Jesper L Andersson, Junqian Xu, Saad Jbabdi, Matthew Webster, Jonathan R Polimeni, David C Van Essen, Mark Jenkinson, WU-Minn HCP Consortium. Neuroimage, 2013.
5. A neural basis for general intelligence. J Duncan, R J Seitz, J Kolodny, D Bor, H Herzog, A Ahmed, F N Newell, H Emslie. Science, 2000.
6. The lateral occipital complex and its role in object recognition. Grill-Spector, Kalanit, Zoe Kourtzi, and Nancy Kanwisher. Vision research 2001.
7. Fluid intelligence allows flexible recruitment of the parieto-frontal network in analogical reasoning. Preusse, F., Van Der Meer, E., Deshpande, G., Krueger, F. and Wartenburger, I. Frontiers in human neuroscience 2011.
Figures