2460
Multi-Spectral Susceptibility-Weighted Imaging in the Presence of Metallic Hardware1Radiology, Medical College of Wisconsin, Milwaukee, WI, United States
Synopsis
To address the unmet need for metal artifact suppressed susceptibility-weighted imaging (SWI) in neuroradiology, here we introduce a novel approach to the generation of SWI contrasts using conventional (currently available) 3D-multi-spectral imaging (MSI) metal-artifact-suppression sequences. This approach leverages basic spin-echo based 3D-MSI sequences, and then utilizes the inherent spectral information within MSI to develop "phase-contrast" maps that can then used to generate SWI-like contrasts. This new metal-suppressed SWI approach has potential applications in patients with high susceptibility intra-cranial hardware, such as aneurysm clips, vascular shunts, cochlear implants, and fixed dental/orthodontic hardware.
Introduction
Commonly encountered metal devices in neuroimaging include aneurysm clips, flow diverters, vascular shunts, cochlear implants, and permanent dental/orthodontic hardware. When performing MRI examinations in the presence of these devices, significant image artifacts are often induced and often renders exams (at least partially) non-diagnostic.Multi-spectral imaging sequences substantially reduce the dominant MRI artifacts around metal implants1,2. To date, the image contrasts available using MSI have been developed for exclusively orthopaedic applications (i.e. imaging near total joint replacements). Though T1, T2, and diffusion-weighted MSI sequences have been developed, susceptibility-weighted imaging (SWI)3, which is a well-established diagnostic tool, is a key missing contrast in the MSI neuroimaging portfolio.
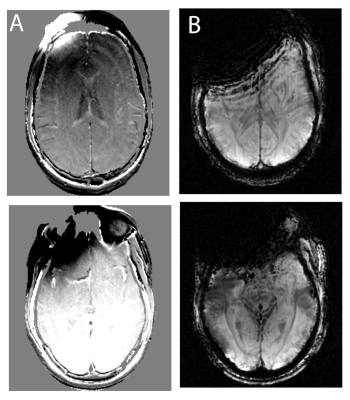
Developing SWI contrast near metal implants is a substantial challenge, as SWI utilizes T2* signal dephasing and in-plane phase accumulation to to build its unique tissue contrast. It is well-known that T2* signal dephasing, which is enabled using gradient-echo pulse-sequences, is the most nefarious MRI artifact induced by metal implants. As a result, gradient-echo sequences (and SWI by extension) are avoided at all costs whenever metallic instrumentation is near the imaging field of view (FOV). Figure 1 demonstrates this problem, displaying the SWI artifact encountered when a high susceptibility object (cobalt-chromium implant) is placed on the skull within the imaging FOV. The 3D-MSI field maps (A) show the presence of the implant, which substantially degrades the conventional SWI images shown in (B), eliminating the majority of the anterior portion of the brain signal.
To address the unmet need for metal artifact suppressed SWI MRI, here we introduce a novel approach to the generation of SWI contrasts using conventional (currently available) 3D-MSI sequences. This approach leverages basic spin-echo based 3D-MSI sequences, further utilizing the inherent spectral information within MSI to develop "phase-contrast" maps that can then used to generate SWI-like contrasts.
Methods and Results
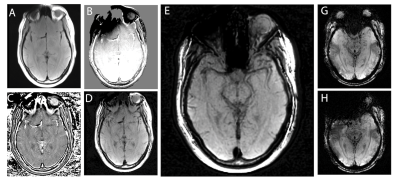
Fundamental SWI methodology typically generates phase-accumulation maps from T2*-weighted GRE acquisitions, high-pass filters the phase maps, and then multiplies the magnitude T2* image with the filtered phase raised to an exponential factor. The MSI-based approach presented in this work takes a similar approach, but instead of the phase-accumulation maps, MSI-based spectral perturbation maps are leveraged. These maps are well-established leveraging magnitude spectral-bin image data to estimate local off-resonance values4. Native T2* dephasing contrast is fundamentally incompatible with imaging metallic instrumentation (which is readily apparent in Figure 1), so alternative magnitude contrast options are required. As an initial exploration of potential contrasts, both T1 and intermediate-weighted 3D-MSI were utilized for magnitude weighting in the present demonstration.Figure 2 provides a demonstration and explanation of the of the 3D-MSI SWI approach utilized in the present work.
Test images were acquired at 3 Tesla using T1-weighted (TE = 8.4 ms, TR = 647.2 ms, ETL = 12) and T2-weighted (TE = 85 ms, TR = 3500ms, ETL 40 and flow-compensation) 3D-MSI with 24 spectral bins with 1kHz spacing. Conventional SWI imaging was derived from multi-echo GRE images using 3-direction flow compensation (TEmean = 23 ms, TR =37.8 ms, 5 echoes). All images were acquired with 1x1 mm2 in-plane resolution across 28 axial 4mm slice-encoded sections. Acquisition times for the images were 5 minutes for the 3D-MSI SWI and 4 minutes for the conventional multi-echo SWI acquisition.
Demonstration images were acquired on a single healthy volunteer subject with a large cobalt-chromium acetabular implant component placed on their right forehead region, and then again without the device in place. The test subject provided written consent into a locally-approved IRB protocol.
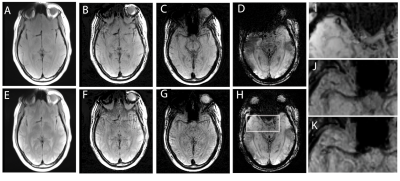
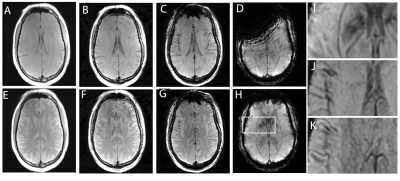
Figures 3 and 4 present the T1 weighted MSI, T2 weighted MSI, and conventional approaches for SWI processing. Each figure provides sample images for a separate axial slice.
Discussion
It is clear from the results in Figures 2-4 that the 3D-MSI SWI approached can recover signal that is lost near metal implants when using conventional SWI approaches. It is also clear from the zoomed in images of Figures 3 and 4 that the derived SWI contrast is variable between the T1 and T2-weighted SWI and the conventional T2*/T1 weighted SWI. This is an expected result that will require further investigation, particularly in pathological cases. Since both 3D-MSI approaches can clearly enhance vasculature, as expected in an SWI image, they are also likely to detect off-resonance localized variations (such as bleeds or calcifications). However, the two approaches do not have the hallmark T2* weighted background contrast that is expected in SWI images. Future work will explore the combined use of T1 and T2 weighted MSI to generate a fused contrast that is more indicative of the conventional SWI approach. This is a reasonable approach to enhance the 3D-MSI based contrast, as a conventional MSI-based neurological exam will typically collect both the T1 and T2-weighted MSI contrasts. Another future direction of exploration will leverage more advanced approaches for 3D-MSI field map generation, which move beyond the simple center-of-mass spectral approach utilized in this study. Several recent publications5,6 have demonstrated new and improved mechanisms of field map generation that are less likely to contain underlying tissue contrast contamination in the resulting field maps.Acknowledgements
This research was supported by National Institute of Health (NIH) grant R21EB030123.References
[1] Koch, K. M., Lorbiecki, J. E., Hinks, R. S., & King, K. F. (2009). A multispectral three-dimensional acquisition technique for imaging near metal implants. Magnetic Resonance in Medicine, 61(2), 381–390. http://doi.org/10.1002/mrm.21856
[2] Lu, W., Pauly, K. B., Gold, G. E., Pauly, J., & Hargreaves, B. (2009). SEMAC: Slice encoding for metal artifact correction in MRI. Magnetic Resonance in Medicine, 62(4), 66–76.
[3] S. Mittal, Z. Wu, J. Neelavalli, and E. M. Haacke. Susceptibility-weighted imaging: Technical aspects and clinical applications, Part 2.American Journal of Neuroradiology, 30(2):232–252, 2009
[4] Koch, K. M., Brau, A. C., Chen, W., Gold, G. E., Hargreaves, B. A., Koff, M., et al. (2011). Imaging near metal with a MAVRIC-SEMAC hybrid. Magnetic Resonance in Medicine, 65(1), 71–82. http://doi.org/10.1002/mrm.22523
[5] Quist, B., Shi, X., Weber, H., & Hargreaves, B. A. (2017). Improved field‐mapping and artifact correction in multispectral imaging. Magnetic resonance in medicine, 78(5), 2022-2034.
[6] Koch, K. M., Koff, M. F., Bauer, T. W., Shah, P. H., Nencka, A. S., Sivaram Kaushik, S., & Potter, H. G. (2017). Off-resonance based assessment of metallic wear debris near total hip arthroplasty. Magnetic Resonance in Medicine, 93(3), 293–10. http://doi.org/10.1002/mrm.26807
Figures