2452
Channel Pruning to Improve Robustness of Pilot Tone-based Cardiac Trigger Detection1The Ohio State University, Columbus, OH, United States
Synopsis
In this work, we develop and apply a method to prune channels from multi-coil Pilot Tone (PT) data. The method suppresses contributions from noisy or corrupt channels and thus improves the quality of the PT-driven cardiac signal. The method, called Spectrum Optimized Channel pruning (SOC), is based on maximizing the ratio of the signal energy in the frequencies that correspond to physiological motions to the signal energy in the frequencies that are outside the physiological motions. Using data from 15 subjects, we highlight that the proposed channel pruning method improves the quality of the extracted cardiac signal.
Introduction
Pilot Tone (PT) is an emerging technology that can improve cardiovascular MRI (CMR) by enabling contactless extraction of physiological signals [1]. If proven robust, PT can replace electrocardiogram (ECG), navigator echoes, and even self-gating. PT operates by continuously transmitting an off-resonant narrowband RF signal during acquisition from a dedicated transmitter placed in close proximity to the subject. The transmitted signal is modulated by the physiological motions and is received by all elements (channels) of the receive coil array. Based on their position, not all channels encode the physiological signal (e.g., respiration or cardiac motion) to the same extent. Also, noise and interference can vary significantly across channels. Here, we propose a channel pruning procedure that can suppress contributions from channels with a low-quality signal.Methods
Spectrum Optimized Channel pruning (SOC) is based on the idea of beamforming, which has been recently applied to MRI for suppressing signal from areas outside the region-of-interest (ROI) [2]. For the implementation of SOC, first, the PT data is Fourier transformed along the time dimension to construct a matrix $$$\boldsymbol{P}\in\mathbb{C}^{T\times C}$$$, where $$$T$$$ is the number of time samples and $$$C$$$ is the number of PT channels. Second, $$$\boldsymbol{P}$$$ is decomposed into two non-overlapping matrices, i.e., $$$\boldsymbol{P} = \boldsymbol{P}_F + \boldsymbol{P}_I$$$. Here, $$$\boldsymbol{P}_F$$$ represents frequencies-of-interest, while $$$\boldsymbol{P}_I$$$ represents interference frequencies. Non-zero entries in $$$\boldsymbol{P}_F$$$ encompass likely frequencies of the physiological motions, i.e., -4 Hz to 4 Hz, while the non-zero entries in $$$\boldsymbol{P}_I$$$ capture frequencies that represent noise or distortion, i.e., below -6 Hz and above 6 Hz. From $$$\boldsymbol{P}_F$$$ and $$$\boldsymbol{P}_I$$$, Hermitian symmetric matrices $$$\boldsymbol{V}$$$ and $$$\boldsymbol{U}$$$ are computed using:$$\boldsymbol{V}=\boldsymbol{P}^\textsf{H}_F \boldsymbol{P}_F\dots (1)$$
$$\boldsymbol{U}=\boldsymbol{P}^\textsf{H}_I \boldsymbol{P}_I\dots (2)$$
For $$$k=1,2,\dots,C$$$, $$$\boldsymbol{w}_k\in\mathbb{C}^{C\times 1}$$$ and $$$\lambda_k\geq 0$$$ are calculated using a generalized eigen decomposition of $$$\boldsymbol{U}$$$ and $$$\boldsymbol{V}$$$, given in Eq. 3, and the signal-to-interference ratio (SIR) is calculated using $$$\boldsymbol{U}$$$, $$$\boldsymbol{V}$$$, and $$$\boldsymbol{w}_k$$$ using Eq. 4. The channel combining weights, $$$\boldsymbol{w}_k$$$, are sorted in descending order of $$$\lambda_k$$$ such that $$$\boldsymbol{w}_1$$$ maximizes the SIR criterion. Then, for $$$n\leq C$$$, weights $$$\boldsymbol{w}_1\dots\boldsymbol{w}_n$$$ are used to create $$$n$$$ virtual channels from $$$C$$$ physical channels. For example, $$$\boldsymbol{Pw}_k$$$ generates the $$$k^\text{th}$$$ virtual channel.
$$\boldsymbol{V}\boldsymbol{w}_k=\lambda_k\boldsymbol{U}\boldsymbol{w}_k\dots (3)$$
$$\text{SIR}(\boldsymbol{w}_k) = \frac{\boldsymbol{w}_k^{\textsf{H}}\boldsymbol{V}\boldsymbol{w}_k}{\boldsymbol{w}_k^{\textsf{H}}\boldsymbol{U}\boldsymbol{w}_k}\dots (4)$$
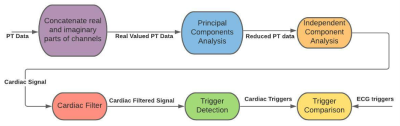
To obtain cardiac triggers, the PT data was passed through the pipeline outlined in Figure 1. To convert the complex-valued channels to real-valued data, the real and imaginary parts of the multi-coil PT data were concatenated. For dimensionality reduction, principal components analysis (PCA) was performed. Independent component analysis (ICA) was then performed to separate respiratory and cardiac signals. The ICA process was repeated five times to increase reliability. Across the five trial runs, the ICA channel with the highest peak in the 0.5 Hz to 3 Hz range was selected as the cardiac signal. The cardiac signal was filtered using a 0.5 Hz – 3 Hz bandpass filter to reduce noise. The approach outlined in [3] was used to determine the correct orientation of the cardiac signal. The cardiac triggers were extracted using peak detection. The resulting cardiac triggers were then evaluated using ECG data as a reference.
Fifteen healthy subjects were scanned on a 1.5T MRI scanner (MAGNETOM Avanto, Siemens Healthcare, Erlangen Germany) anywhere from 30 to 600 s using a gradient echo (GRE) based 4D flow sequence. ECG and PT data were acquired simultaneously. The sampling rate for all ECG scans was 2.5 milliseconds, and the sampling rate for PT was between 4.2 and 4.8 ms. Significant RF interference or bulk motion distortion was observed in four of the fifteen datasets. The acquired PT dataset was processed twice, once with SOC ($$$n=10$$$) and once without.
Results
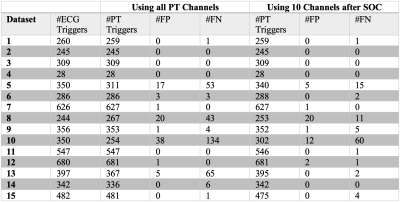
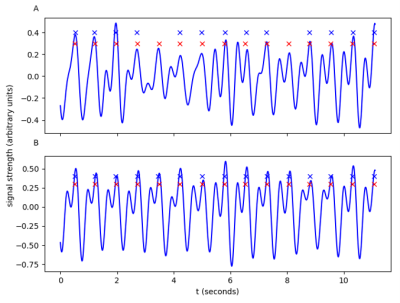
The results are summarized in Figure 2. A false negative indicates the presence of an ECG trigger without an accompanying PT trigger, and a false positive indicates a PT trigger without an accompanying ECG trigger. For Subjects 5, 8, 10, and 13, using SOC led to a considerable reduction in the number of false positives and false negative triggers. For other Subjects, the performances with and without SOC were comparable. Figure 3 shows an example where SOC enhances the quality of the extracted cardiac signal, providing improvement in trigger detection.Discussion
Our preliminary results show that SOC can improve the performance of trigger detection by suppressing contributions from noisy or corrupt channels. For the datasets already performing well, the proposed channel pruning did not negatively impact the trigger detection. In Figure 3, a visual inspection shows a much clearer cardiac signal and more consistent trigger extraction after the channel pruning. During trigger detection, a cutoff value was used to identify peaks. This cutoff parameter provided a trade-off between false positives and false negatives and could be further optimized. All processing was done retrospectively. In future studies, we will apply SOC for the prospective processing of the PT data.Conclusion
This preliminary study demonstrates that channel pruning can improve the reliability of cardiac trigger detection from multi-coil PT data.Acknowledgements
This work was supported by NIH R01HL151697.References
[1] M. Bacher, Cardiac Triggering Based on Locally Generated Pilot-Tones in a Commercial MRI Scanner: A Feasibility Study. MS Thesis, Graz University of Technology, 2017
[2] D. Kim, S. F. Cauley, K. S. Nayak, R. M. Leahy, and J. P. Haldar, “Region- optimized virtual (ROVir) coils: Localization and/or suppression of spatial regions using sensor-domain beamforming,” Magn. Reson. Med., vol. 86, no. 1, 2021
[3] C. Chen, Y. Liu, O. P. Simonetti, and R. Ahmad, “Automatic Extraction and Sign Determination of Respiratory Signal in Real-Time Cardiac Magnetic Resonance Imaging,” In 2020 IEEE 17th International Symposium on Biomedical Imaging (ISBI) 2020 Apr 3 (pp. 1-4).
Figures