2436
Recurrent Variational Inference for fast and robust reconstruction of accelerated FLAIR MRI in Multiple Sclerosis1Department of Biomedical Engineering & Physics, Amsterdam UMC, University of Amsterdam, Amsterdam, Netherlands, 2Department of Anatomy & Neurosciences, MS Center Amsterdam, Amsterdam Neuroscience, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, Netherlands, 3Department of Medical, Health and Neuropsychology, Leiden University, Leiden, Netherlands, 4Delft University of Technology, Delft, Netherlands
Synopsis
Robustness when applying Deep Learning methods to clinical data is crucial for accurate high-resolution reconstructions while having fast inference times. We propose the Cascades of Independently Recurrent Variational Inference Machine (CIRVIM), targeting deep unrolled optimization and enforcing data consistency for further robustness. We quantify contrast resolution of seven and half times prospectively undersampled FLAIR MRI without fully-sampled center containing Multiple Sclerosis lesions. The proposed scheme reduces inference times by a factor of 6 compared to Compressed Sensing. Lesion contrast resolution improves by approximately 13% while preserving spatial detail with enhanced sharpness compared to more blurred results of other presented methods.
Purpose
To reconstruct out-of-training-distribution high-resolution images containing Multiple Sclerosis lesions with sufficient contrast resolution and fast inference times.Introduction
In MRI high acceleration factors can be achieved with Compressed Sensing (CS) [Donoho, et al. (2006)] to facilitate the slow acquisition times, but the reconstruction times are relatively slow. Machine Learning (ML) can vastly reduce inference times while producing high-resolution images. Sampling significantly fewer data points results in kspace results in an aliased image in the image domain. The barrier when applying an ML method for dealiasing and denoising MRI data in the clinical setting is its robustness in generating accurate results. The main challenge is the generalization to new data, where it is unknown how to restore the fully sampled image given sparsely sampled measurements. This is known as the inverse problem of accelerated-MRI reconstruction. ML methods learn how to solve it from the data instead of applying a predefined sparsifying transform as in CS. We assess the robustness of an unrolled scheme targeting optimization by gradient descent to solve the inverse problem of accelerated-MRI reconstruction. We use pretrained networks and compare them to CS for evaluating fast high-resolution reconstructions of out-of-training-distribution clinical data of Multiple Sclerosis patients with known white matter lesions.Methods
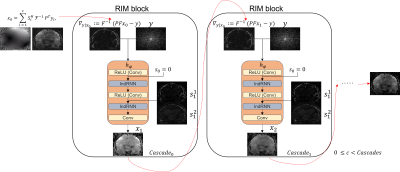
The Recurrent Inference Machine [Lønning, K. et al. (2019)] solves the inverse problem of accelerated-MRI reconstruction through a sequence of alternating convolutional and recurrent layers. To reduce the model’s complexity and, thus, inference time, we chose the Independently Recurrent Neural Network (IndRNN) [Li, S. et al. (2018)] as an option for the recurrent layers and built an Independently Recurrent Inference Machine (IRIM). Its recurrent nature allows it to capture long-range dependencies through time steps, so robustness is assessed by increasing them. Nevertheless, training RNNs with a large number of time-steps is challenging due to often out-of-memory errors by vanishing or exploding gradients. For that purpose, we selected a general variational scheme of cascades and built RIM blocks sequentially connected. We call this method Cascades of Independently Recurrent Variational Inference Machine (CIRVIM) (Figure 1).We trained networks on fully sampled 3D 3.0T T1-weighted brain data (details on [Lønning, K. et al. (2019)]), as well as 2D FLAIR brain data (details on [Muckley et al., 2020]), retrospectively undersampled up to ten times on a Gaussian distribution. The training and validation sets consisted of 3150 slices for the T1 dataset and 6750 for the FLAIR dataset. The IRIM and CIRVIM models were built with 64 channels and 8 time-steps. The cascades were tuned with selected values varying between 1 for building an IRIM and 4, 6, and 8 for building larger CIRVIM models. Parallel-Imaging Compressed Sensing (PICS) reconstructions were done using the BART toolbox with l1-regularization set to 0.05 and 60 iterations (Uecker et al., 2015) (Figure 2). All experiments were performed on an NVidia Tesla V100 GPU with 32GB of memory.
The test dataset contained 18 3D FLAIR scans of relapsing remitting Multiple Sclerosis patients with known white matter lesions, prospectively undersampled with a factor of 7.5 on a Variable-Density Poisson disk distribution. Data were acquired on a 3.0T Philips Ingenia scanner (Philips Healthcare, Best, The Netherlands) in Amsterdam UMC within the scope of a more extensive, ongoing study. The local ethics review board approved this study, and patients provided informed consent prior to imaging. A fully-sampled reference scan was also acquired and used to estimate coil sensitivity maps using the caldir method of the BART toolbox. We quantified the performance by measuring contrast resolution (CR) of the lesion relative to surrounding white matter after reconstruction: $$$CR\ =\frac{S_{lesion}\ -\ S_{WM\:surrounding\:lesion}}{S_{lesion}\ +\ S_{WM\:surrounding\:lesion}}$$$.
Results
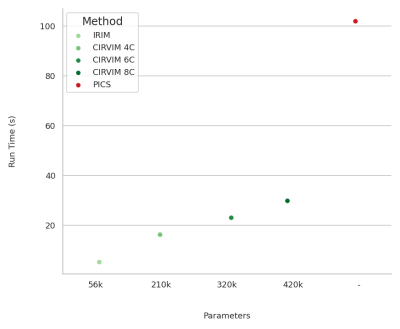
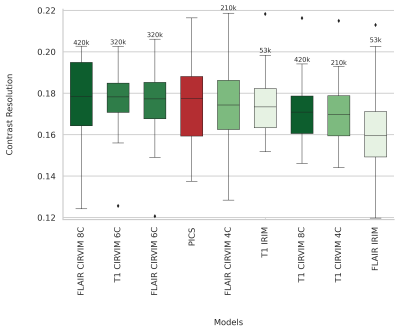
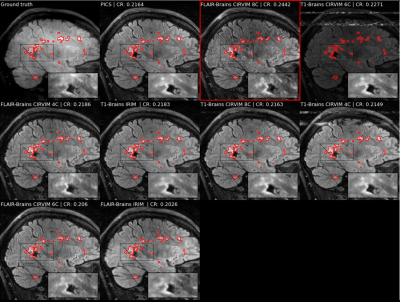
The highest scoring method on measuring contrast resolution was the CIRVIM with eight cascades trained on the FLAIR data (Figure 3). The IRIM was the fastest method requiring ~6 seconds for reconstructing a 32-coils volume from the test set with 224 slices and matrix size 223$$$\times$$$163. The largest CIRVIM with eight cascades was six times slower, while PICS was sixteen times slower than the IRIM. PICS scored fourth-best with overall more blurred results (Figure 4). The other CIRVIMs scored comparably to PICS, with overall sharper results but not up to the level of the model with the eight cascades. Quantitative evaluation of an example reconstructed slice showed an improvement in contrast resolution of approximately 13% of the FLAIR-trained CIRVIM compared to PICS (Figure 4). The second-highest scoring model (T1-trained IRIM) showed artifacts, while other reconstructions showed more apparent blurring.Discussion/Conclusion
The Cascades of Independently Recurrent Variational Inference Machine (CIRVIM) target robustness through cascades for learning optimization by gradient descent. The CIRVIM built with eight cascades and trained on FLAIR data scored highest on measuring contrast resolution for reconstructing Multiple Sclerosis lesions with an undersampling factor of 7.5x. The models trained on the T1 dataset appeared to be robust in reconstructing FLAIR data. The updated inputs through the cascades can enhance performance, thus generalization to clinical data containing pathologies unseen during training. Our method showed promising generalization capabilities, preserving spatial detail on reconstructing MS lesions with enhanced sharpness in contrast to a more blurred result by CS, as well as reducing the inference times up to six times.Acknowledgements
This publication is based on the STAIRS project under the TKI-PPP program. The collaboration project is co-funded by the PPP Allowance made available by Health~Holland, Top Sector Life Sciences & Health, to stimulate public-private partnerships.
Also, we would like to thank Marike van Lingen and Kevin Pancras for their contribution.
References
Donoho, D. L. (2006) ‘Compressed sensing’, IEEE Transactions on Information Theory, 52(4), pp. 1289–1306. doi: 10.1109/TIT.2006.871582.
Lønning, K. et al. (2019) ‘Recurrent inference machines for reconstructing heterogeneous MRI data’, Medical Image Analysis, 53, pp. 64–78. doi: 10.1016/j.media.2019.01.005.
Li, S. et al. (2018) ‘Independently Recurrent Neural Network (IndRNN): Building A Longer and Deeper RNN’, Proceedings of the IEEE Computer Society Conference on Computer Vision and Pattern Recognition, (1), pp. 5457–5466. doi: 10.1109/CVPR.2018.00572.
Muckley, M. J. et al. (2020) ‘State-of-the-Art Machine Learning MRI Reconstruction in 2020: Results of the Second fastMRI Challenge’. Available at: http://arxiv.org/abs/2012.06318.
Uecker, M. et al. (2015) ‘Berkeley Advanced Reconstruction Toolbox’, Proc. Intl. Soc. Mag. Reson. Med., 23.Figures