2432
Design and fabrication of portable, low-field MR relaxometer for clinical measurement of volemic status1HST, MIT, Cambridge, MA, United States, 2DMSC, MIT, Cambridge, MA, United States
Synopsis
A low-field portable MR-based sensor was fabricated for the acquisition of clinical T2 relaxometry measurements in skeletal muscle. We have previously reported methods for designing low-field permanent magnet array configurations with varying sensitive region profiles. The newly constructed array has a sensitive region 15-20mm from the surface of the magnet; field maps from the constructed magnet show good alignment with simulated fields. The exclusion of subcutaneous fat tissue in the sensitive region will improve sensitivity to fluid shifts within the skeletal muscle.
Background
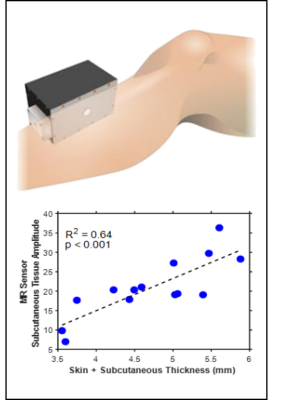
Worldwide there are over 2.5 million patients with end stage kidney disease who depend on life-sustaining dialysis treatments. The intermittent nature of dialysis exposes patients to wide variations between fluid overload on non-dialysis days and fluid depletion after hemodialysis sessions1. Fluid overload has been linked with cardiovascular events and mortality2,3. Fluid depletion results in intradialytic hypotension, which occurs in up to 75% of hemodialysis patients and can cause nausea, vomiting, and cramping. Long-term volume overload and poorly managed ultrafiltration rates are also common4,5. There is currently no quantitative standard for fluid status assessment that informs physicians or patients of their volemic status. An adequate assessment of fluid state in routine clinical practice is, therefore, an unmet need6. Methods for quantification of fluid status, including bioelectric impedance analysis, plasma osmolality, and isotope dilutions, are known to have clinically relevant limitations7. We aim to establish the magnetic resonance (MR) based measurement as a rapid quantitative method for fluid assessment. We have developed a portable, single-sided MR sensor to collect relaxometry metrics from skeletal muscle, where previous studies have indicated excess fluid accumulates8.We have previously demonstrated a single-sided MR relaxometer (SSR) that is capable of acquiring T2 relaxometry measurements from the lower leg of patients. In a clinical study with dialysis patients, our prototype MR sensor was compared against a clinical MRI scanner. Clinical MRI was able to able to detect differences between hyper- and hypo- volemic patients from lower leg skeletal muscle, but the SSR was unable to distinguish between those groups due to the presence of unequal amounts of subcutaneous fat in the sampling volume. SSR measurements did show statistically significant changes for each patient before and after hemodialysis. The SSR used in the clinical study was designed with permanent magnets to have a static magnetic field of 0.27 T with a saddle-shaped homogeneous region 2-7 mm from the surface of the magnet. Physically, this region includes both muscle and subcutaneous fat compartments when placed against a patient’s leg. The inclusion of subcutaneous fat in the measurement confounds the signal from the interstitial muscle space, as shown in Fig. 18. The purpose of this work is to construct a modified portable permanent magnet array and RF coil sensor capable of acquiring signal from only the skeletal muscle of a leg and sufficiently sensitive enough to be used for clinical measurements.
Methods
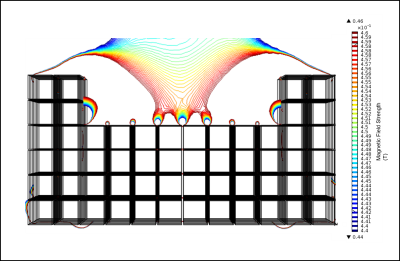
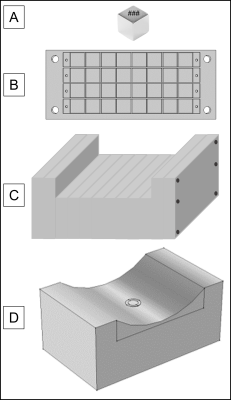
Previously presented results detail the methods and outcomes for designing a portable magnet array using finite element analysis with COMSOL Multiphysics software. The resulting design, shown in Fig. 2, has a homogeneous region 12-15m above the surface of the array. The array was constructed from 1/2" cube N52 neodymium magnets9. Each serialized magnet was individually flux tested; only magnets within a single standard deviation of the median flux density were included in the array assembly to increase field homogeneity, the rest were discarded. Custom aluminum frames were fabricated to secure the magnets in the proper orientation, shown in Fig. 3. Each frame was field mapped with a hall probe and gaussmeter prior to array assembly. The frame slices were assembled and secured on aluminum rods and the net magnetic field of the completed assembly was mapped. A surface RF coil (N=8, diameter = 16mm) was constructed and placed on the surface of the magnet assembly to obtain a T2 signal from the sensitive region.Results
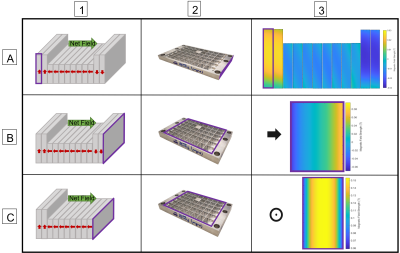
Low-field permanent Halbach magnet array configurations were modeled in COMSOL Multiphysics until a simulated magnetic field profile met our requirements for homogenous region. The array configuration that achieves the desired field consists of 448 1/2" cube permanent magnets with two 1mm thick iron yokes. Magnets were arranged in specially constructed aluminum frames. Each frame was field mapped with a hall probe prior to assembly to ensure magnets were secured in the correct orientation and identify any variations in frame field homogeneity; representative field maps are shown in Fig 4. Analysis showed insignificant variation across rows of assembled frames, indicating high magnetic field homogeneity. Individual frames were assembled and fit with an RF coil, the final field was mapped and a T2 signal acquired.Discussion
A low-field, single-sided, portable MR-based sensor was constructed for the acquisition of clinical T2 relaxometry measurements in skeletal muscle. The depth of the homogeneous region, 12-15mm above the surface of the RF coil allows for the exclusion of subcutaneous fat in the sweet spot – which will improve sensitivity to fluid shifts within the skeletal muscle. Over 400 cube neodymium magnets were selected for field uniformity and used in array construction. Modular sections of the array, consisting of 32-48 magnet cubes were assembled in custom aluminum frames and field mapped to ensure a high level of slice homogeneity. The frames were then stacked and secured with aluminum rods to complete the array and field mapped with a hall probe and gaussmeter. A surface RF coil was fit on the middle of the array and T2 measurements were obtained using a CPMG sequence. This portable low-field magnet design will enable clinical assessment of fluid status without the need for full-scale clinical MRI imaging.Acknowledgements
Funding: NSF GRFP
Cima Lab: Michael Cima, Haley Higgenbotham, Hannah Jackson, Erin Rousseau, Kriti Subramanyam, & Wendy Brown
Cima Lab Alumni: Chris Frangieh, Ashvin Bashyam
MIT Machine Shop: Andy Gallant
References
[1] Hecking M, Karaboyas A, Antlanger M, Saran R, Wizemann V, Chazot C, et al. Significance of interdialytic weight gain versus chronic volume overload: consensus opinion. Am J Nephrol. 2013;38(1):78–90
[2] Kalantar-Zadeh K, Regidor DL, Kovesdy CP, Van Wyck D, Bun- napradist S, Horwich TB, et al.: Fluid retention is associated with car- diovascular mortality in patients undergoing long-term hemodialysis. Circulation 119:671–679, 2009
[3] Wizemann V, Wabel P, Chamney P, Zaluska W, Moissl U, Rode C, et al.: The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transplant 24:1574–1579, 2009
[4] J.Q. Jaeger, R.L. Mehta, Assessment of dry weight in hemodialysis an overview, J. Am. Soc. Nephrol. 10 (1999) 392–403.
[5] J.E. Flythe, S.E. Kimmel, S.M. Brunelli, Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality, Kidney Int. 79 (2011) 250–257.
[6] S. Ishibe, A.J. Peixoto, Methods of Assessment of Volume Status and Intercompartmental Fluid Shifts in Hemodialysis Patients: Implications in Clinical Practice, in: Semin. Dial., Wiley Online Library, 2004: pp. 37–43. doi:10.1111/j.1525-139X.2004.17112.x.
[7] S. McGee, W.B. Abernethy III, D.L. Simel, Is this patient hypovolemic?, Jama. 281 (1999) 1022–1029.
[8] L.Colucci, K. Corapi, M. Li, X. Parada, A Allegretti, H Lin, D. Ausiello, M. Rosen, M.J. Cima, Fluid assessment in dialysis patients by point-of-care magnetic relaxometry; Science Translational Medicine, 10.1126/scitranslmed.aau1749, Vol 11, Issue 502, 2019/07/24
[9] A. Bashyam, M. Li, M.J. Cima, Design and experimental validation of Unilateral Linear Halbach magnet arrays for singlesided magnetic resonance, J. Magn. Reson. (2018).
Figures