2430
A Transceiver Coil for improved in ovo Imaging of the Chorioallantoic Membrane at 7T1Institute of Medical Physics and Radiation Protection, TH Mittelhessen University of Applied Sciences, Giessen, Germany, 2Center for Tumor Biology and Immunology, Core Facility for Small Animal MRI, Marburg, Germany, 3Department of Pharmaceutics and Biopharmaceutics, Philipps-University of Marburg, Marburg, Germany, 4Department of Diagnostic and Interventional Radiology, Philipps-University of Marburg, Marburg, Germany
Synopsis
The chorioallantoic membrane (CAM) model is a simple and low-cost model that allows screening of a large number of pharmacological samples. It is used in preclinical research as an intermediate step between in vitro and in vivo experiments and does not require approval by the animal experiment ethics committee. Latest studies show that MRI is a suitable imaging modality for evaluating drug delivering systems with the CAM-model. However, appropriate hardware for high-resolution MR imaging of the CAM model is still missing. To fill this gap, we designed, constructed, and evaluated a transceiver solenoid coil for in ovo imaging at 7T.
Introduction
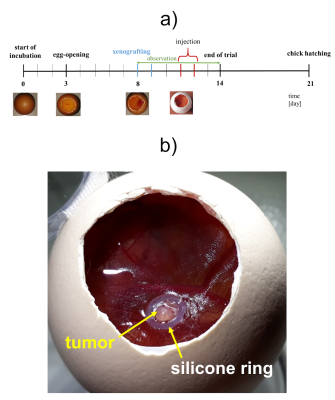
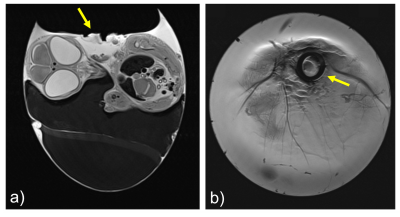
The chorioallantoic membrane (CAM) (Fig 1) of the developing chick embryo is an extraembryonic membrane mediating gas and nutriment exchanges until hatching.1,2 The absence of an evolved immune system and the presence of a dense capillary network of the CAM model enables the study of angiogenesis and antiangiogenesis3-5, wound healing6, tissue engineering7, biomaterials and implants8-10, and biosensors11. Recent studies have show that MRI is a suitable imaging modality for evaluating nanoscale drug delivery systems (DDS) in cancer therapy through its ability to provide high-resolution images with great soft tissue contrast.12-14 However, small-bore MRI scanners in particular are mainly equipped with dedicated coils for small animals, which are highly limited to image decapitated eggs in an upright position. With this limitation, in ovo imaging is usually performed with relatively large volume coils, e.g., quadrature birdcages.2-14To take in ovo MR imaging to the next level of sensitivity and spatial resolution, we have developed and validated a dedicated transceiver coil to improve signal-to-noise ratio (SNR) and provide a practical and easy-to-use coil design for CAM model imaging studies.
Methods
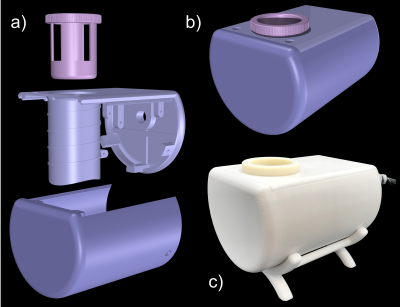
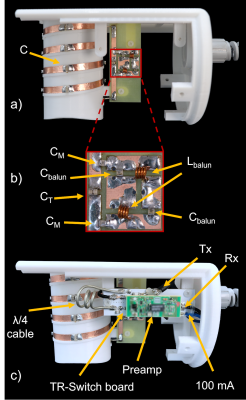
(i) Design and Bench Measurement: An in-built cylindrical structure along with standoffs for TR-switch and balun circuit enclosed in a semi-cylindrical model was constructed and 3D-printed (Fortus450mc, Stratasys Ltd., Eden Prairie, USA) in poly-carbonate plastic (Fig 2). Copper coil was wrapped four times around the cylindrical coil former (diam. = 56$$$\,$$$mm) to create a solenoid (Fig 3). The solenoid coil was tuned to 300.42$$$\,$$$MHz by alternately distributing 4 and 7$$$\,$$$pF capacitors along the copper path. Impedance matching to 50$$$\,$$$Ohm was achieved by symmetrically adding two 6.8$$$\,$$$pF capacitors in series. A lumped lattice-type LC balun contributed towards common mode rejection between coil and TR-switch circuit. Capacitors and inductors with values 6.8$$$\,$$$pF and 22$$$\,$$$nH, respectively, were used in the lattice balun. An in-house built TR-switch circuit was used to achieve the desired isolation between the transmitter and receiver, allowing them to utilize the same coil. The coil was validated with RF bench-level measurements such as unloaded-to-loaded Q-ratio, tuning, matching, and TR-switch performance.(ii) Data acquisition: A 7T small-bore MRI scanner (Clinscan 70/30, Bruker, Ettlingen, Germany) was used for CAM-Model imaging. PD-weighted GRE images of a fertilized egg were acquired to obtain the SNR of the constructed coil (TE/TR/FA = 300$$$\,$$$ms / 3.5$$$\,$$$ms / 25$$$^{\circ}$$$, in-plane res. = 0.5x0.5$$$\,$$$mm2, slice thickness = 0.1$$$\,$$$mm, BW = 260$$$\,$$$Hz/pixel, avg. = 2). Pixel-wise SNR was calculated from the raw data by dividing the signal values by the standard deviation of the noise only image. We compared the SNR of the constructed coil to the SNR obtained from scanner's built-in birdcage coil. Initial CAM-Model images with xenograft tumor tissue were carried out using a TSE sequence (TE/TR = 3000$$$\,$$$ms / 45$$$\,$$$ms, in-plane res. = 156x156$$$\,$$$µm2, slice thickness = 0.6$$$\,$$$mm, BW = 130$$$\,$$$Hz/pixel, avg. = 6).
Results
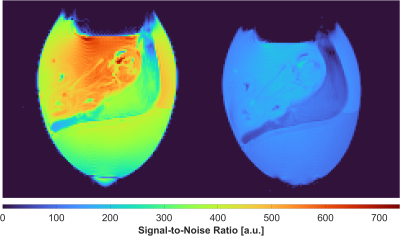
The S11 reflection coefficient of the coil input was -25$$$\,$$$dB. The unloaded-to-loaded Q-ratio was measured to be QUL/QL = 210/50 = 4.2. In transmit mode, the TR-switch provided an S21 isolation of -29$$$\,$$$dB between Tx and Rx. In receive mode, the isolation between Tx and the coil was -29$$$\,$$$dB.The constructed coil outperformed the scanner's birdcage coil by a factor of 3.1 in SNR (Fig 4). The initial in ovo images (Fig 5) obtained from the constructed coil demonstrate the ability to acquire high-resolution images of the CAM, including the xenograft tumor tissue, and the chick embryo.
Discussion
A transciever solenoid coil for in ovo imaging of the chorioallantoic membrane was designed, constructed, and evaluated. The semi-cylindrical coil housing with the removable egg holder has proven valuable for imaging decapitated eggs in an upright position. The close-fitting coil design provided a 3.1-fold improvement in SNR compared to the small-bore scanner's birdcage. The improved SNR could be traded for acquiring high-resolution images of the chorioallantoic membrane. Initially acquired high-quality structural images suggest, that the coil would enable growth monitoring of xenograft tumor tissue in DDS studies using the CAM model.Conclusion
The constructed solenoid coil enables image acquisition with up to 3.1-fold greater SNR compared to the built-in birdcage coil of the small animal scanner. This should permit high-resolution imaging of the CAM in DDS studies.Acknowledgements
References
1. Vargas, Angelica, et al. "The chick embryo and its chorioallantoic membrane (CAM) for the in vivo evaluation of drug delivery systems." Advanced drug delivery reviews 59.11 (2007): 1162-1176.
2. Ribatti, Domenico. "The chick embryo chorioallantoic membrane (CAM) assay." Reproductive toxicology 70 (2017): 97-101.
3. Ribatti, Domenico, et al. "The chick embryo chorioallantoic membrane as a model for in vivo research on angiogenesis." International Journal of Developmental Biology 40.6 (2004): 1189-1197.
4. Ribatti, Domenico, et al. "Chorioallantoic membrane capillary bed: A useful target for studying angiogenesis and anti‐angiogenesis in vivo." The Anatomical Record: An Official Publication of the American Association of Anatomists 264.4 (2001): 317-324.
5. Richardson, Mary, and Gurmit Singh. "Observations on the use of the avian chorioallantoic membrane (CAM) model in investigations into angiogenesis." Current Drug Targets-Cardiovascular and Hematological Disorders 3.2 (2003): 155-185.
6. Ribatti, Domenico, et al. "The chick embryo chorioallantoic membrane as an in vivo wound healing model." Pathology-Research and Practice 192.10 (1996): 1068-1076.
7. Borges, Joerg, et al. "Chorioallantoic membrane angiogenesis model for tissue engineering: a new twist on a classic model." Tissue engineering 9.3 (2003): 441-450.
8. Zwadlo-Klarwasser, G., et al. "The chorioallantoic membrane of the chick embryo as a simple model for the study of the angiogenic and inflammatory response to biomaterials." Journal of Materials Science: Materials in Medicine 12.3 (2001): 195-199.
9. Valdes, T. I., D. Kreutzer, and F. Moussy. "The chick chorioallantoic membrane as a novel in vivo model for the testing of biomaterials." Journal of Biomedical Materials Research: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 62.2 (2002): 273-282.
10. Klueh, Ulrike, et al. "Ex ova chick chorioallantoic membrane as a novel model for evaluation of tissue responses to biomaterials and implants." Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 67.3 (2003): 838-843.
11. Valdes, T. I., et al. "Ex ova chick chorioallantoic membrane as a novel in vivo model for testing biosensors." Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 67.1 (2003): 215-223.
12. Preis, Eduard, et al. "The chorioallantoic membrane as a bio-barrier model for the evaluation of nanoscale drug delivery systems for tumour therapy." Advanced Drug Delivery Reviews (2021).
13. Zuo, Zhi, et al. "High‐resolution MRI analysis of breast cancer xenograft on the chick chorioallantoic membrane." NMR in biomedicine 28.4 (2015): 440-447.
14. Hafner, Susanne, et al. "High‐contrast magnetic resonance imaging and efficient delivery of an albumin nanotheranostic in triple‐negative breast cancer xenografts." Advanced Therapeutics 2.11 (2019): 1900084.
Figures