2427
Elucidating dynamic anaerobic metabolism in obligate anaerobes with living cells1Radiology and Pathology, Massachusetts General HospitalMassachusetts General Hospital, Harvard Medical School, Charlestown, MA, United States, 2Massachusetts Host-Microbiome Center, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, United States, 3Institut Pasteur, Université de Paris, Paris, France, 4Centro de Investigación en Enfermedades Tropicales, Facultad de Microbiología, Universidad de Costa Rica, San Jose, Costa Rica, 5Laboratory of the Pathogenesis of Bacterial Anaerobes, Institut Pasteur, Université de Paris, Paris, France, 6Radiology, The University of Texas Southwestern Medical Center, Dallas, TX, United States
Synopsis
Anaerobic microbial metabolism drives critical functions within global ecosystems, host-microbiota interactions, and industrial applications, yet remains ill-defined. We resolved dynamic metabolism in living cells of the anaerobic pathogen Clostridioides difficile using High-Resolution Magic Angle Spinning (HRMAS) Nuclear Magnetic Resonance (NMR) spectroscopy to inform genome-scale predictions of cellular metabolism. Analyses leveraged the sensitivity of 13C NMR spectroscopy to simultaneously track cellular carbon and nitrogen flow from fermentable 13C and 15N-labeled substrates. We illustrate a versatile approach to elaborate complex anaerobic metabolism for clinical, scientific, and industrial applications.
Introduction
Obligate anaerobes comprise the majority of species in the mammalian gut microbiota and include pathogens such as Clostridioides difficile, but despite their global importance, many anaerobes and their metabolic systems remain poorly characterized. High-resolution magic angle spinning (HRMAS) NMR spectroscopy supports studies of real-time metabolism in living cells.1-3 HRMAS NMR is particularly suited to the study of anaerobes as the sealed rotor chamber can maintain an anaerobic environment.3 Furthermore, detailed studies of metabolism can be achieved with a low input biomass of cells.3 When coupled with cellular metabolism of uniformly carbon-13 (13C) labeled substrates, HRMAS NMR’s improved sensitivity enables definitive tracking of carbon flow through complex metabolic pathways.In contrast, tracking weak NMR-active nuclei through cellular metabolism has been more difficult. Amino-group nitrogen, in particular, has been challenging to detect given that nitrogen-15 (15N) produces 15-fold less signal than 13C.4 However, NMR J-coupling between 13C and covalently bound 15N induces predictable patterns of nuclear spin-spin splitting in 13C signals,5-6 enabling detection of the weaker 15N nucleus in the more sensitive 13C NMR spectrum. We leveraged this concept of NMR physics to enable the detection of bonds formed between 15N and 13C in living cells to track simultaneous carbon and nitrogen flow in C. difficile’s complex metabolism.
Methods
To investigate the progression of carbon source metabolism, we measured HRMAS NMR time series of proton (1H) and 13C NMR spectra from living C. difficile cells. Cultures were grown in Modified Minimal Media (MMM) that replaced a given natural abundance carbon source with its uniformly-labeled carbon-13 isotopologue: [U-13C]glucose, L-[U-13C]proline, or L-[U-13C]leucine, nutrients known to drive rapid pathogen growth in vivo.7 Analyses tracked the molecular context of 13C atoms to resolve dynamic metabolism relative to the consumption of preferred substrates. Analyses used fermentable 13C- and 15N-labeled substrates to track single carbon and nitrogen flow through cellular metabolism. The use of isotopically substituted compounds enables high-resolution time series of anaerobe metabolism within complex nutrient conditions, including ones encountered in vivo.7Results
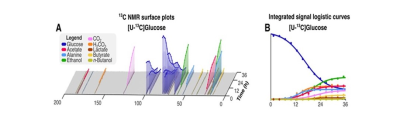
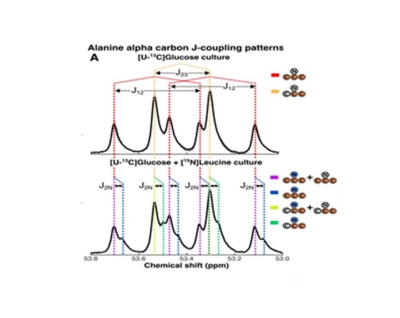
HRMAS NMR of C. difficile grown with [U-13C]glucose (Fig. 1A-B, dark blue plots) identified small molecule metabolites from progressive oxidative and reductive phases of metabolism. [13C]acetate and [13C]alanine, oxidative products from of [U-13C]glucose metabolism, were first detected at 7 hours (Fig. 1A-B, red and light blue plots, respectively). Reductive glucose metabolism began at 10 hours with production of [13C]ethanol (Fig. 1A-B, green plots), followed by [13C]butyrate (Fig. 1A-B, yellow plots) and [13C]lactate at 18 hours (Fig. 1A-B, brown plots), and n-[13C]butanol at 35 hours (Fig. 1A-B, light green plots).HRMAS 13C NMR of C. difficile grown with [U-13C]glucose and natural abundance leucine revealed [2,3-13C]alanine and [U-13C]alanine in a 1:1 ratio (Fig. 2A), indicating substantial assimilation of 12CO2 with [U-13C]acetate. C. difficile grown in the presence of [U-13C]glucose and [15N]leucine showed 15N-induced splitting of the 13C peaks associated with alanine’s alpha carbon (Fig. 2B, blue and green lines) and mixed populations of [15N]alanine and [14N]alanine (Fig. 2B, purple and yellow lines). A small up-field chemical shift isotope effect due to the presence of 15N was also detected. Though [15N]leucine represented only 33% of amino-group nitrogen in the starting media, 57±4% of [13C]alanine carried the 15N isotope, confirming enriched transfer of the 15N amino group from fermented [15N]leucine to [13C]alanine (Fisher’s Exact Test, p=0.001). We illustrate integrated use of HRMAS 13C NMR and genome-scale metabolic modeling to define concurrent and progressive recruitment of oxidative and reductive fermentation pathways, with associated electron transport and energy-generating systems, in the obligate anaerobe C. difficile.
Discussion
We present the first high-resolution studies of real-time metabolism in living obligately anaerobic cells. Our results demonstrate the utility of HRMAS NMR to sustain an anaerobic reaction chamber for continuous monitoring of anaerobic microbial metabolism. Our use of 15N13C J-coupling to amplify less NMR-sensitive 15N nuclei with more sensitive 13C NMR presents a conceptual foundation for the simultaneous evaluation of multiple atomic species to determine their biological and chemical importance, including ones that could be detected through covalent bonding with other NMR-active nuclei such as 1H or 17O. When combined with genome-scale metabolic modeling, HRMAS 13C NMR time series informed complex cellular metabolism to the level of supporting pathways, electron transport and redox systems, and gene-level targets.Conclusion
In aggregate, this analytic approach can support evaluation of other dynamic processes, including microbial responses to antibiotics, or optimization of conditions to produce industrially important chemicals from different input feedstocks. Integrated use of live cell HRMAS 13C NMR with dynamic metabolic modeling provides a greatly improved approach to define cellular-scale anaerobic metabolism to support diverse applications.Acknowledgements
We received financial support and resources from the following : the National Institutes of Health grant R01AI153653, National Institutes of Health grant P30DK056338, BWH Precision Medicine Institute, National Institutes of Health grant S10OD023406, National Institutes of Health grant R21CA243255, National Institutes of Health grant R01AG070257, and the MGH A. A. Martinos Center for Biomedical ImagingReferences
1. L. L. Cheng et al., Quantitative neuropathology by high resolution magic angle spinning proton magnetic resonance spectroscopy. Proceedings of the National Academy of Sciences of the United States of America. 94, 6408-6413 (1997).
2. M. Tilgner, T. S. Vater, P. Habbel, L. L. Cheng, High-Resolution Magic Angle Spinning (HRMAS) NMR Methods in Metabolomics. Methods Mol Biol 2037, 49-67 (2019).
3. M. T. Judge et al., Continuous in vivo Metabolism by NMR. Front Mol Biosci 6, 26 (2019).
4. S. G. Patching, NMR-Active Nuclei for Biological and Biomedical Applications. Journal of Diagnostic Imaging in Therapy 3, 7-48 (2016).
5. R. Nieto, F. Cruz, J. M. Tejedor, G. Barroso, S. Cerdan, Origin of the ammonia used for mitochondrial citrulline synthesis as revealed by 13C-15N spin coupling patterns observed by 13C NMR. Biochimie 74, 903-911 (1992).
6. Lapidot, A. Gopher, Quantitation of metabolic compartmentation in hyperammonemic brain by natural abundance 13C-NMR detection of 13C-15N coupling patterns and isotopic shifts. Eur J Biochem 243, 597-604 (1997).
7. S. Gencic, D. A. Grahame, Diverse Energy-Conserving Pathways in Clostridium difficile: Growth in the Absence of Amino Acid Stickland Acceptors and the Role of the Wood-Ljungdahl Pathway. J Bacteriol 202, (2020).
Figures