2425
DEXSY Reveals Water Exchange Coupled to Activity1National Institute of Child Health and Human Development, Potomac, MD, United States, 2National Institute of General Medical Sciences, Potomac, MD, United States, 3Department of Clinical Neurosciences, Wellcome Centre for Integrative Neuroimaging, FMRIB, University of Oxford, Oxford, United Kingdom, 4National Institute of Neurological Disorders and Stroke, Potomac, MD, United States
Synopsis
Currently, no MRI method exists to non-invasively and absolutely measure cellular activity. One approach is to quantify steady-state transmembrane water exchange rates. Diffusion Exchange Spectroscopy (DEXSY) non-invasively and directly encodes for exchange of endogenous components. Using DEXSY-based methods implemented on a low-field, high-gradient MR system, we show in viable ex vivo neonatal mouse spinal cord samples that the rate at which water exchanges across cell membranes decreases drastically after perturbation known to induce persistent membrane depolarization. We also show that the exchange rate recovers to normal values after perturbations known to restore membrane potential.
Introduction
Membrane potential is like a battery being maintained at a steady charge by ATPase pumps while being used to drive various forms of cellular activity. The battery can drain faster than it is charged, leading to sustained, steady-state depolarization (ssD) of membrane potential. In the central nervous system (CNS), persistent reduction in Na+/K+-ATPase activity, such as through energy failure (hypoxia, hypoglycemia) e.g., during stroke, can cause spreading depolarization (SD), reducing the membrane potential from -70mV to -10mV in an “all-or-none” fashion.1 After the initial spreading depolarization has passed, steady state depolarization (ssD) remains until the tissue recovers. ssD can occur under physiological conditions as well, for instance ssD is a part of long-term potentiation and neural plasticity.2 Current measurements of ssD are either invasive (intracellular recording of membrane potential) or they only follow relative changes and require a baseline reading (electrocorticography or noninvasive electroencephalography and diffusion MRI). Although directly checking the "charge" of cells in tissue is difficult, perhaps the steady-state exchange of water, which is measurable with MR, can report the charge. Dynamic contrast enhanced (DCE) MR experiments have revealed steady-state transmembrane water transport linked to Na+/K+-ATPase pump activity in animal cells.3 Bai et al., reported steady-state transmembrane water exchange rates decreased by 50% after ouabain was used to block Na+/K+-ATPase pump activity in organotypic CNS.4 Williamson, et al. (2019) developed real-time diffusion exchange spectroscopy (DEXSY) MR methods to study water homeostasis in ex vivo neonatal mouse spinal cords.5 We use the same approach here. Samples are kept viable during MR measurements, as confirmed by recordings of motoneuronal electrical activity after dorsal root stimulation at the end of the experiment.5 Another abstract submitted to this (ISMRM 2022) conference shows our methods are sensitive to active water exchange. Continuing that work, in this study we test the hypothesis that water exchange is linked to membrane activity.Methods
MR measurements were performed on viable “live” ex vivo neonatal mouse spinal cords at 13.79 MHz with a low-field single-sided magnet (PM-10 NMR MOUSE, Magritek)6 and custom-made RF probe and solenoid coil (Fig. 1).5 Diffusion was encoded on sub-micron length scales and sub-millisecond timescales with spin echoes in the presence of a g=15.3 T/m static gradient (SG).7 SG diffusion measurements (see Ref. [5]) and SG diffusion exchange spectroscopy (DEXY)-based exchange rate and spin-lattice relaxation rate measurements (as in Ref. [8], with b1+b2=4.5 ms/µm2) were repeatedly acquired (11 minutes per set) to observe real-time changes. During measurements, samples were maintained in a constant circulation of artificial cerebrospinal fluid (aCSF) and gas and at 25°C.Results and Discussion
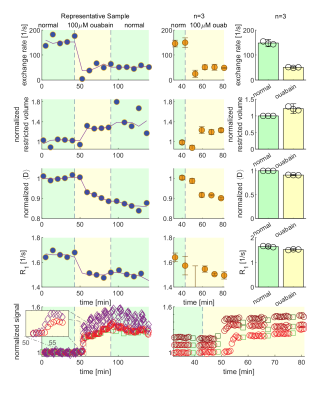
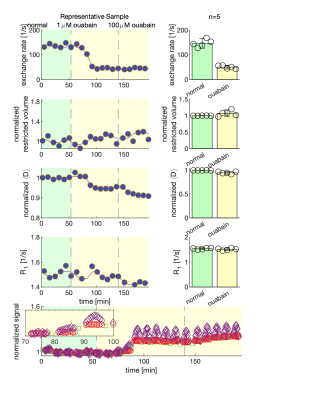
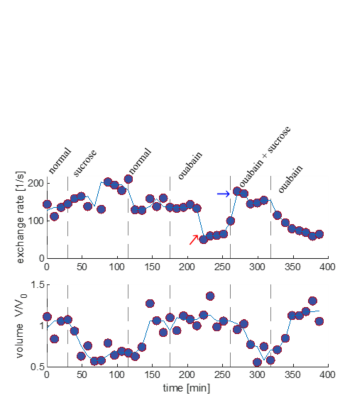
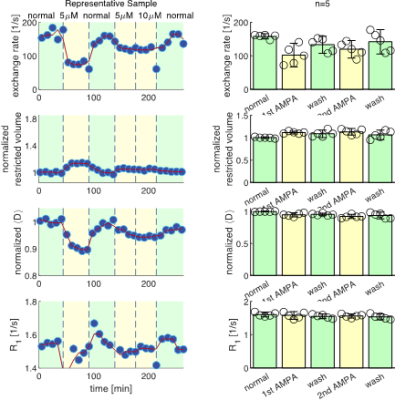
We present data suggesting that exchange rate is linked to ssD. Completely blocking Na+/K+-ATPase pump activity with 100 μM ouabain caused exchange rate to swiftly decrease to 40 s-1 (Fig. 1). Partially blocking Na+/K+-ATPase pump activity with doses as low as 1 μM also caused exchange rate to decrease to 40 s-1, but after a longer delay (Fig. 2). Ouabain had an “all-or-none”, dose-independent effect on exchange rate. In comparison, Balestrino, et al. (1999), reported that blocking Na+/K+ pump activity with ouabain induced a sustained SD-like effect consistent with ssD in hippocampal slices.9 The SD-like effect showed an “all-or-none” response to ouabain—not a dose response. In Fig. 3 we show that adding 100 mM sucrose (osmolyte) in normal conditions had a small effect on exchange rate, but in ouabain-treated states led to a drastic recovery from 40 s-1 back to 150 s-1, consistent with the literature finding that ouabain-induced SD can be abolished by addition of an osmolyte9. Fig. 4 shows that AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) causes exchange rate to decrease and that the effect is reversible when AMPA is washed out. In comparison, Falgairolle and O’Donovan (2015) showed that AMPA causes a sustained depolarization in the neonatal mouse spinal cord which is reversible upon washout.10 Fig. 4 also shows that a second dose of AMPA had less of an effect, consistent with recycling of receptors conditioning the cells to AMPA.10 In summary, we have found that ouabain and AMPA substantially reduce the exchange rate and that osmolyte addition recovers the exchange rate from the ouabain-reduced state back to a value consistent with the normal state. We also recapitulate findings in the literature showing that ssD is induced by ouabain and AMPA and that ouabain-induced ssD can be abolished by addition of an osmolyte.9,10 The consistency between our findings and the literature circumstantially supports the view that the water exchange rate is coupled to steady state depolarization (ssD).Conclusion
Cellular activity may be non-invasively and absolutely measurable through a coupled process—water exchange—via DEXSY MR. We show that the water exchange rate maintains a stable and repeatable value across samples under normal conditions, suggesting it to be an intrinsic or absolute measure of water exchange in these cells. Exchange rates decrease and recover predictably following perturbations known to cause steady-state depolarization (ssD) and recovery. DEXSY is sensitive and specific to endogenous water exchange and may find utility as a measurement of biological activity for both basic cell biology and medicine.Acknowledgements
NHW was funded by the NIGMS PRAT Fellowship Award # FI2GM133445-01. RR, TXC, and PJB were supported by the IRP of the NICHD, NIH. MF and MJO were funded by the IRP of the NINDS.References
1. G. G. Somjen, "Mechanisms of spreading depression and hypoxic spreading depression-like depolarization," Physiological reviews, vol. 81, no. 3, pp. 1065-1096, 2001.
2. W. F. Boron and E. L. Boulpaep, Medical physiology E-book. Elsevier Health Sciences, 2016.
3. C. S. Springer Jr, "Using 1H2O MR to measure and map sodium pump activity in vivo," Journal of Magnetic Resonance, vol. 291, pp. 110-126, 2018.
4. R. Bai, C. S. Springer Jr, D. Plenz, and P. J. Basser, "Fast, Na+/K+ pump driven, steady‐state transcytolemmal water exchange in neuronal tissue: A study of rat brain cortical cultures," Magnetic resonance in medicine, vol. 79, no. 6, pp. 3207-3217, 2018.
5. N. H. Williamson et al., "Magnetic resonance measurements of cellular and sub-cellular membrane structures in live and fixed neural tissue," Elife, vol. 8, p. e51101, 2019.
6. G. Eidmann, R. Savelsberg, P. Blümler, and B. Blümich, "The NMR MOUSE, a mobile universal surface explorer," Journal of Magnetic Resonance, Series A, vol. 122, no. 1, pp. 104-109, 1996.
7. N. H. Williamson et al., "Unprecedented diffusion weighting and exchange resolution of cellular and sub-cellular structures in live and fixed neural tissue," in Proceedings of the 28th annual International Society of Magnetic Resonance in Medicine (ISMRM), 2020.
8. N. H. Williamson et al., "Real-time measurement of diffusion exchange rate in biological tissue," Journal of Magnetic Resonance, vol. 317, p. 106782, 2020.
9. M. Balestrino, J. Young, and P. Aitken, "Block of (Na+, K+) ATPase with ouabain induces spreading depression-like depolarization in hippocampal slices," Brain research, vol. 838, no. 1-2, pp. 37-44, 1999.
10. M. Falgairolle and M. J. O’Donovan, "Pharmacological investigation of Fluoro-Gold entry into spinal neurons," PloS one, vol. 10, no. 6, p. e0131430, 2015.
Figures