2424
Hyperpolarising 13C-glucose with an endogenous labile photoinduced radical1Cambridge University, Cambridge, United Kingdom, 2General Electric Healthcare, Chalfont St Giles, United Kingdom
Synopsis
The use of the endogenous molecule alpha-ketoglutarate (α-KG) as a non-persistent radical is examined to hyperpolarise 13C-glucose. A radical build-up curve was obtained and the maximum radical concentration was calculated to be 29 ± 3 mM. The microwave irradiation frequency was then examined to optimise the polarisation transfer between the electron on α-KG and glucose. A dissolution was successfully carried out and a hyperpolarised 13C spectrum of 13C-glucose was obtained.
Introduction
Hyperpolarised magnetic resonance imaging (MRI) of metabolites is usually carried out using persistent radicals, however, when used in clinic, they require removal. Here we examine the use of a non-persistent radical formed on alpha-ketoglutarate (α-KG), to hyperpolarise glucose. The use of trimethylpyruvic acid as a non-persistent radical has been carried out1, however, the safety profile of this molecule is as yet unknown. Whereas, α-KG is endogenous and already used in the clinic2,3 and has already been successfully used with lactic acid4. Therefore, this non-persistent radical was used to examine hyperpolarised glucose, with a view to use this for metabolic imaging.Methods and Results
A sample of α-KG (1 M) and 1-13C-glucose (5 M) was prepared and irradiated using ultraviolet-visible (UV-vis) light, for a cumulative time of 500 s, over 10 time points, and an ESR spectrum was recorded at each point. The radical concentration was calculated by comparing the double integral to a calibration curve using the ESR (Fig. 1). The radical build-up curve showed a maximum concentration of α-KG radical to be 29 ± 3 mM, with a build-up time constant of 70 s.To examine the polarisation of glucose whilst in the presence of α-KG, a second sample was prepared using 1-13C,U-2H-glucose (5 M) and α-KG (1 M). Ten, 6 µL beads were flash frozen in N2(l) and irradiated with UV-vis light for 200 seconds using a Dymax Bluewave200 broadband source. Solid-state DNP experiments were performed using a home built fluid path4 to load the sample into a 7 T cryogen free polariser5, to find the optimum microwave frequency (Fig. 2).
The sample was then irradiated with the optimum microwave frequency and dissolution was carried out into a 9.4 T MR scanner. The 13C MR spectrum shown in figure 3, shows a hyperpolarised glucose 13C signal.
Discussion
The use of 13C hyperpolarised glucose has already been discussed in analysing the aerobic glycolysis of glioblastoma6, breast cancer cells7 and also as a 13C MR probe8. However, with the use of persistent radicals and prohibitively short T1 relaxation time, this is yet to be observed in clinic as a lot of the hyperpolarised signal has decayed before the measurement has started. From the data presented here we have shown that glucose can be hyperpolarised using the labile radical formed on the endogenous molecule, α-KG. This not only demonstrates the versatility of this molecule as a labile radical but also the large polarisation obtained for 13C-glucose.Conclusion
Using the endogenous molecule α-KG, we obtained a large radical concentration of 29 ± 3 mM, which produced a hyperpolarised 13C glucose signal. As persistent radicals no longer need to be used, the process in which they need to be removed is not required, therefore shortening the time between dissolution and acquisition, whilst also preserving the T1. This opens the way for transport and storage of hyperpolarised 13C-glucose for human applications.Acknowledgements
No acknowledgement found.References
1. Capozzi, A. et al. Efficient Hyperpolarization of U-(13) C-Glucose Using Narrow-Line UV-Generated Labile Free Radicals. Angew Chem Int Ed Engl. 2019;58:1334-1339
2. Jeppsson A, Ekroth R, Friberg P, Kirnö K, Milocco I, Nilsson FN, Svensson S, Jan Wernerman MD. Renal effects of α-ketoglutarate early after coronary operations. The Annals of thoracic surgery. 1998 Mar 1;65(3):684-90.
3. Kjellman U, Björk K, Ekroth R, Karlsson H, Nilsson F, Svensson G, Jagenburg R, Wernerman J. α-Ketoglutarate for myocardial protection in heart surgery. The Lancet. 1995 Mar 4;345(8949):552-3.
4. Gaunt AP, Lewis JS, Hesse F, Cheng T, Marco-Rius I, Brindle KM, Comment A. Labile photo‐induced free radical in alpha‐ketoglutaric acid: a universal endogenous polarizing agent for in vivo hyperpolarized 13C magnetic resonance. Angewandte Chemie.
5. Cheng T, Gaunt AP, Marco‐Rius I, Gehrung M, Chen AP, van der Klink JJ, Comment A. A multisample 7 T dynamic nuclear polarization polarizer for preclinical hyperpolarized MR. NMR in Biomedicine. 2020 May;33(5):e4264.
6. Mishkovsky M, Gusyatiner O, Lanz B, Cudalbu C, Vassallo I, Hamou MF, Bloch J, Comment A, Gruetter R, Hegi ME. Hyperpolarized 13 C-glucose magnetic resonance highlights reduced aerobic glycolysis in vivo in infiltrative glioblastoma. Scientific reports. 2021 Mar 11;11(1):1-9.
7. Harris T, Degani H, Frydman L. Hyperpolarized 13C NMR studies of glucose metabolism in living breast cancer cell cultures. NMR in Biomedicine. 2013 Dec;26(12):1831-43.
8. Kurhanewicz J, Vigneron DB, Ardenkjaer-Larsen JH, Bankson JA, Brindle K, Cunningham CH, Gallagher FA, Keshari KR, Kjaer A, Laustsen C, Mankoff DA. Hyperpolarized 13C MRI: path to clinical translation in oncology. Neoplasia. 2019 Jan 1;21(1):1-6.
Figures
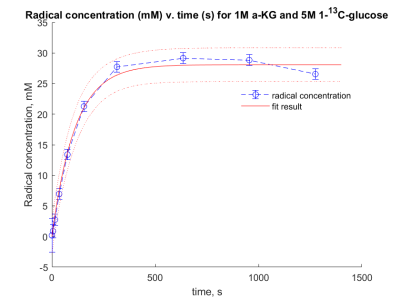
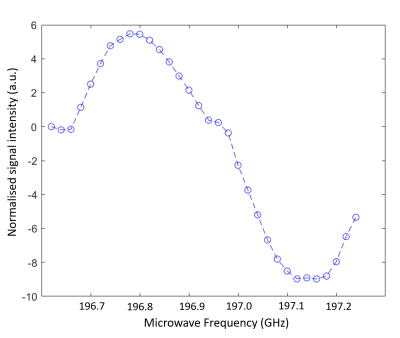
Figure 2 – Microwave spectrum of photo-irradiated α-KG (1 M) in the presence of U-13C,7-2H-glucose (5 M). Measurements were performed at 7 T and 1.3 K using microwave frequency modulation. Microwave frequency was modulated with a sweep width of 56 MHz and an amplitude of 2 kHz.
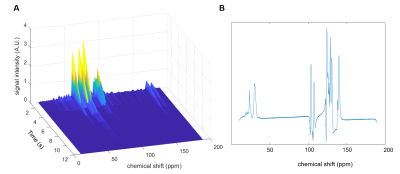
Figure 3 – A) Hyperpolarised U-13C-glucose signal decay time course acquired with a surface coil and a repetition every 1.5 s with an approximate flip angle of 65°. B) shows the first spectrum after completion of injection of the sample into the scanner.